Question: Need help The figure below is from a paper published by Reimers and Dahn in the Journal of the Electrochemical Society 139, 2091 (1992). In
Need help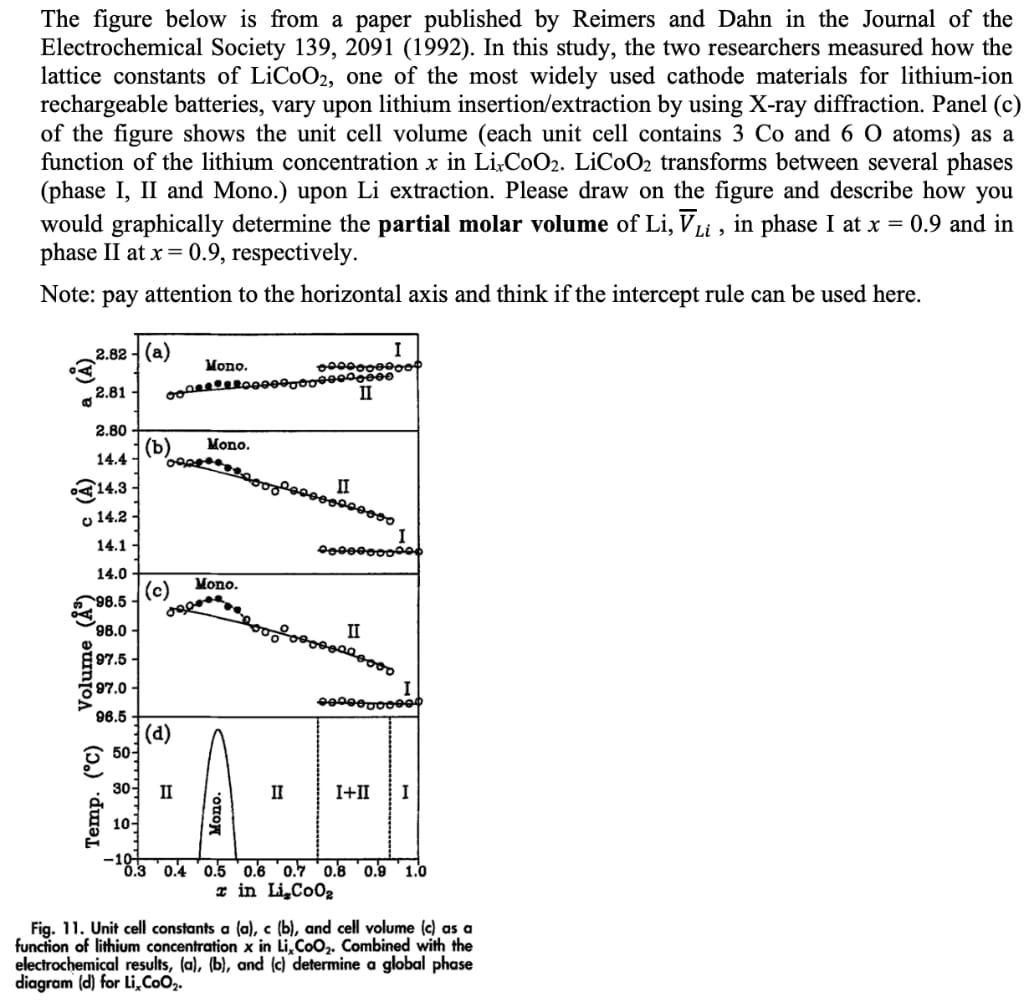
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


