Question: Need help to understand this problem. Would love an explanation please. The bicarbonate buffering system in the blood can be described by the following set
Need help to understand this problem. Would love an explanation please.
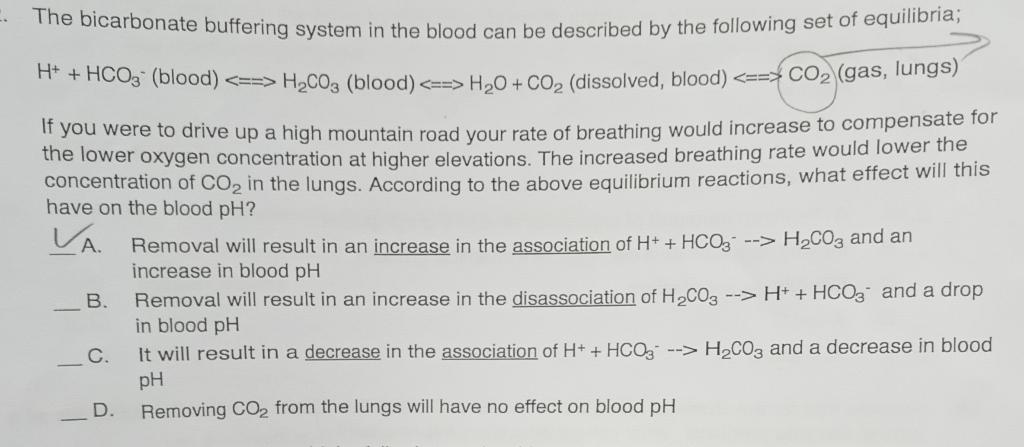
The bicarbonate buffering system in the blood can be described by the following set of equilibria; H++HCO3(blood) H2CO3 (blood) H2O+CO2 (dissolved, blood) CO2 (gas, lungs) If you were to drive up a high mountain road your rate of breathing would increase to compensate for the lower oxygen concentration at higher elevations. The increased breathing rate would lower the concentration of CO2 in the lungs. According to the above equilibrium reactions, what effect will this have on the blood pH ? A. Removal will result in an increase in the association of H++HCO3>H2CO3 and an increase in blood pH B. Removal will result in an increase in the disassociation of H2CO3H++HCO3 and a drop in blood pH - C. It will result in a decrease in the association of H++HCO3H2CO3 and a decrease in blood pH D. Removing CO2 from the lungs will have no effect on blood pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


