Question: need help w part B and C What pressure is required to achieve a CO2 concentration of 6.80102M at 20C ? Express your answer to
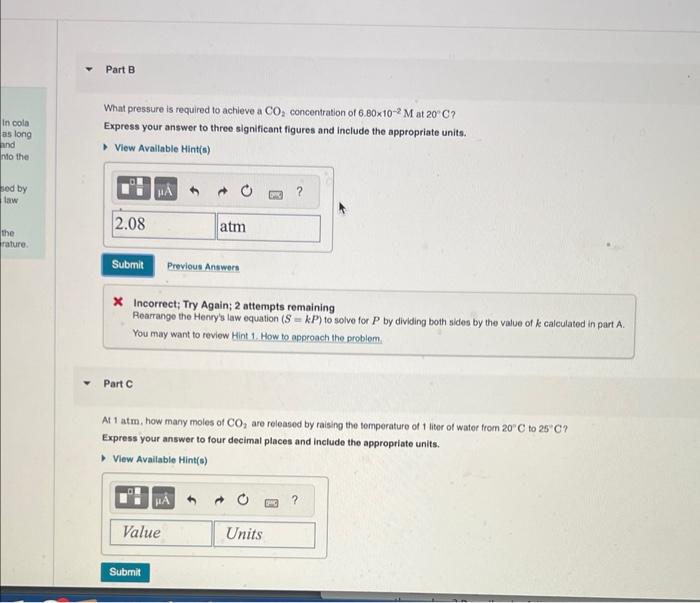
What pressure is required to achieve a CO2 concentration of 6.80102M at 20C ? Express your answer to three significant figures and include the appropriate units. * Incorrect; Try Again; 2 attempts remaining Resrrange the Henry's law equation (S=kP) to solve for P by dividing both sides by the value of k calculated in part A. You may want to review Hint 1 . How to approach the problem. Part C At 1 atm, how many moles of CO2 are released by raising the temperature of 1 liter of water from 20C to 25C ? Express your answer to four decimal places and include the appropriate units. What pressure is required to achieve a CO2 concentration of 6.80102M at 20C ? Express your answer to three significant figures and include the appropriate units. * Incorrect; Try Again; 2 attempts remaining Resrrange the Henry's law equation (S=kP) to solve for P by dividing both sides by the value of k calculated in part A. You may want to review Hint 1 . How to approach the problem. Part C At 1 atm, how many moles of CO2 are released by raising the temperature of 1 liter of water from 20C to 25C ? Express your answer to four decimal places and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


