Question: need help with both homeworks problems please help Consider the reaction 2A(9) = 2B(9) + C(9) For this reaction at 25.0C, the value of Kis

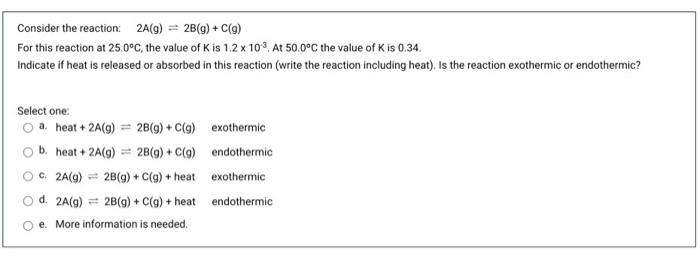
Consider the reaction 2A(9) = 2B(9) + C(9) For this reaction at 25.0C, the value of Kis 8.868. Calculate K for the following reaction at 25.0C. 2B(g) + C(q) = 2A(9) A. 0.11 B. 8,87 C. None of these D. 17.74 E. 10.00 Consider the reaction: 2A(g) = 2B(g) + C(g) For this reaction at 25.0C, the value of Kis 1.2 x 103 At 50.0C the value of Kis 0.34 Indicate if heat is released or absorbed in this reaction (write the reaction including heat). Is the reaction exothermic or endothermic? Select one: a. heat + 2A(9) = 2B(9) + C(q) exothermic b. heat + 2A(9) = 2B(9) + C(9) endothermic C. 2A(g) = 2B(9) + C(q) + heat exothermic d. 2A(g) = 2B(9) + C(g) + heat endothermic e. More information is needed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


