Question: Need help with finding rate constant for number 3 and not sure how to do number 4. please help! Note: tx/10 values can be measured
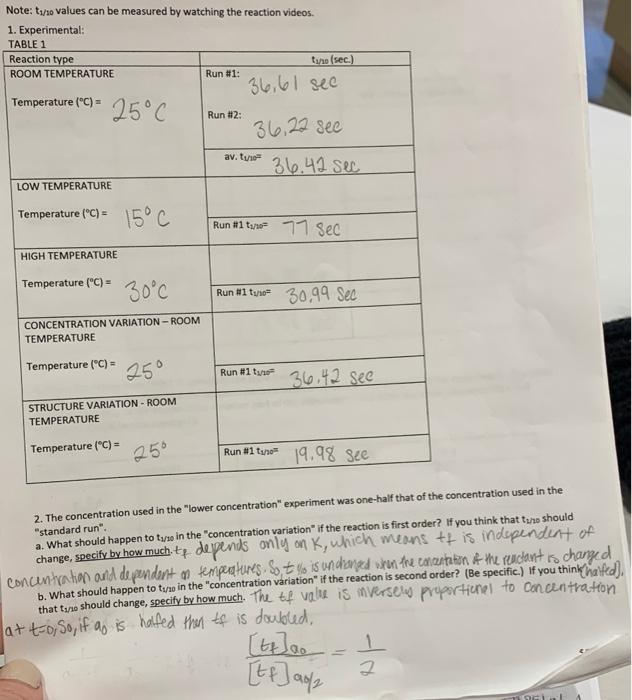
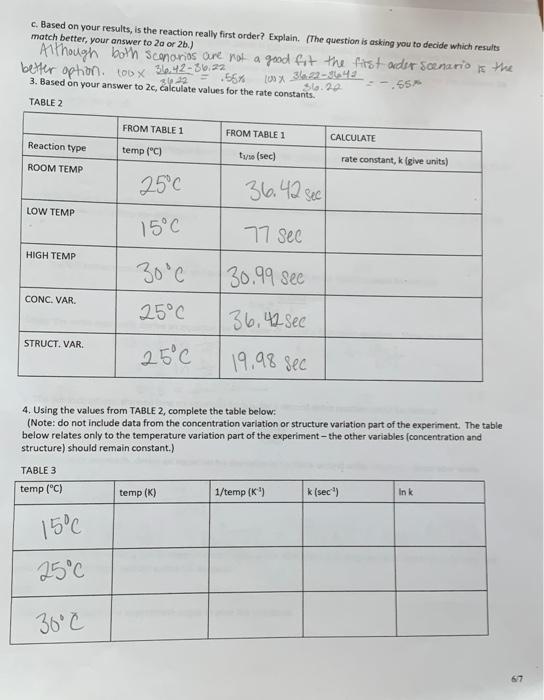
Note: tx/10 values can be measured by watching the reaction videos. 1. Experimental: TABLE 1 Reaction type tano (sec.) ROOM TEMPERATURE Run #1: 36,61 see Temperature (C) - Run #2: 36.22 sec 25C av. trie 36.42 sec LOW TEMPERATURE Temperature (C) = 15C Run #1 tu2 77 Sec HIGH TEMPERATURE Temperature ("C) = 30C Run #1 ture= 30.99 Sec CONCENTRATION VARIATION-ROOM TEMPERATURE Temperature (C) = 25 Run #1 to 36.42 see STRUCTURE VARIATION - ROOM TEMPERATURE Temperature (C) = 25 Run #1 tune 19.98 see 2. The concentration used in the "lower concentration" experiment was one-half that of the concentration used in the "standard run" a. What should happen to t/30 in the concentration variation" if the reaction is first order? If you think that the should change, specify by how much tp depends only on K, which means tt is independent of concentration and dependent on temperatures S to is undoned when the encantation of the reuclant is changed b. What should happen to tuwo in the "concentration variation" if the reaction is second order? (Be specific.) If you think hailed), that tano should change, specify by how much. The tof value is mversele proportionel to concentration atto, So, if ao is halfed then tf is doubled, [telao 2 [ff] ac/ NF c. Based on your results, is the reaction really first order? Explain. (The question is asking you to decide which results match better, your answer to 20 or 2b.) Although both scenanos are not a good fit the first order scenario is the better option. 100 x 36,42-86,22 55% -.55 3623 100% 3.22-642 3. Based on your answer to 2c, calculate values for the rate constants. Slo.20 TABLE 2 FROM TABLE 1 FROM TABLE 1 CALCULATE Reaction type temp (C) tys (sec) rate constant, k lgive units) ROOM TEMP 250 36,42 sec LOW TEMP 15C 77 See HIGH TEMP 30C 30.99 see CONC. VAR. 25C STRUCT. VAR 136,42 see 19,98 sec 25C 4. Using the values from TABLE 2, complete the table below: (Note: do not include data from the concentration variation or structure variation part of the experiment. The table below relates only to the temperature variation part of the experiment - the other variables (concentration and structure) should remain constant.) TABLE 3 temp (C) temp (K) 1/temp (K") k (sec") Ink 15C 25C 30c 67
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


