Question: Need help with flows and composition table Diisopropyl ether (DIPE) is a solvent and fuel additive that is often produced in conjunction with isopropyl alcohol
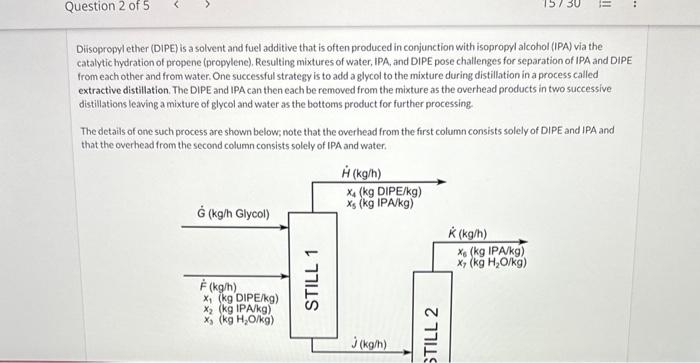
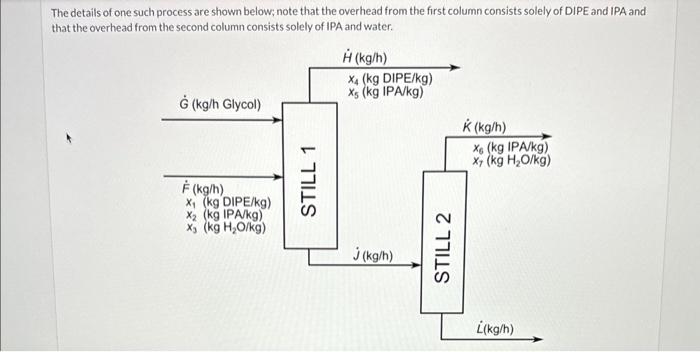
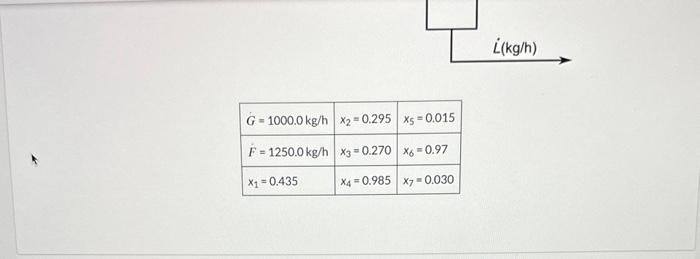
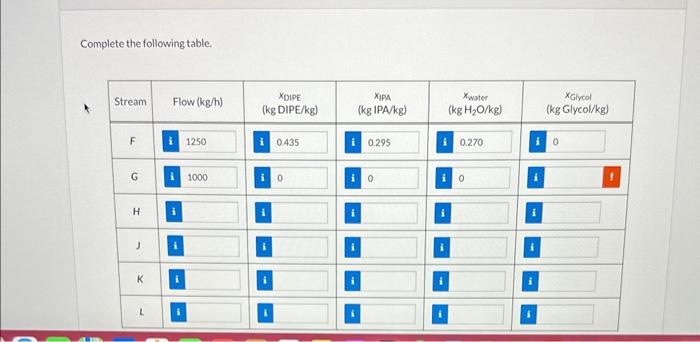
Diisopropyl ether (DIPE) is a solvent and fuel additive that is often produced in conjunction with isopropyl alcohol (IPA) via the catalytic hydration of propene (propylene). Resulting mixtures of water, IPA, and DIPE pose challenges for separation of IPA and DIPE from each other and from water. One successful strategy is to add a glycol to the mixture during distillation in a process called extractive distillation. The DIPE and IPA can then each be removed from the mixture as the overhead products in two successive distillations leaving a mixture of glycol and water as the bottoms product for further processing. The details of one such process are shown below; note that the overhead from the first column consists solely of DIPE and IPA and that the overhead from the second column consists solely of IPA and water. The details of one such process are shown below; note that the overhead from the first column consists solely of DIPE and IPA and that the overhead from the second column consists solely of IPA and water. \begin{tabular}{|l|l|l|} \hlineG=1000.0kg/h & x2=0.295 & x5=0.015 \\ \hlineF=1250.0kg/h & x3=0.270 & x6=0.97 \\ \hlinex1=0.435 & x4=0.985 & x7=0.030 \\ \hline \end{tabular} Complete the following table
Step by Step Solution
There are 3 Steps involved in it
Proceed with similar mass balances ... View full answer

Get step-by-step solutions from verified subject matter experts


