Question: need help with part A,B,C,D and E - Chapter 14 HMWK Q#6 6 of 36 Bel Consell de Part A MISSED THIS? Read Section 145
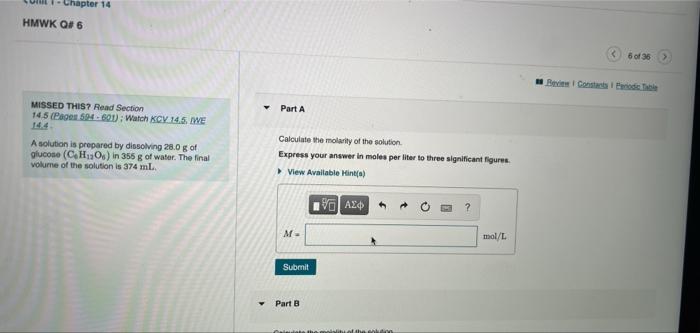
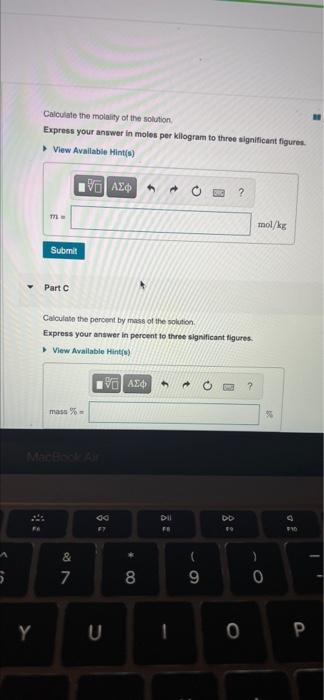
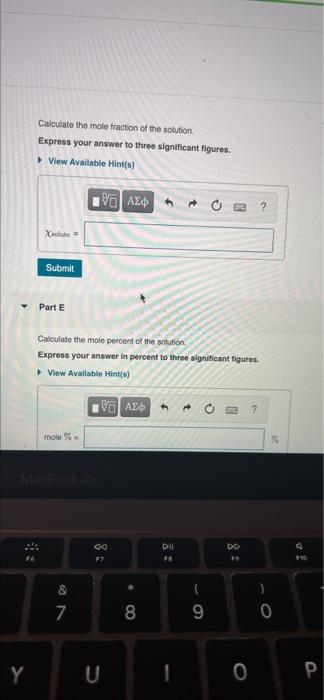
- Chapter 14 HMWK Q#6 6 of 36 Bel Consell de Part A MISSED THIS? Read Section 145 (Pages 594.600) : Watch KCV 14.5. WE 14.4 A solution is prepared by dissolving 28.0 g of glucose (CHO) in 355 g of water. The final Volume of the solution is 374 ml. Calculate the molarity of the solution Express your answer in moles per liter to three significant figures View Available Hints) th ? M mol/L Submit Part B Calculate the molality of the solution Express your answer in moles per kilogram to three significant figures View Available Hints M mol/kg Submit Part C Calculate the percent by mass of the solution Express your answer in percent to three significant figures View Available Hints) mass% do Dil FE DD & * 00 7 8 9 0 O Y U 0 P Calculate the mole fraction of the solution Express your answer to three significant figures. View Available Hints) ? Xente = Submit Part E Calculate the mole percent of the solution Express your answer in percent to three significant figures. View Available Hints) % AED ? molo % co DII DD & S 7 00 8 9 0 Y 1 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


