Question: need help with part C only Liquid propane (C3H8) enters a combustion chamber at 25C at a rate of 1.2 kg/min where it is mixed
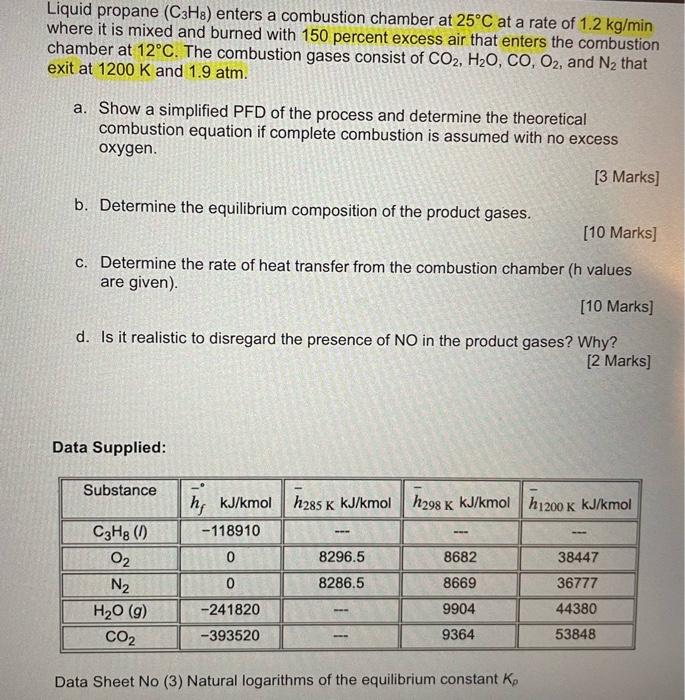
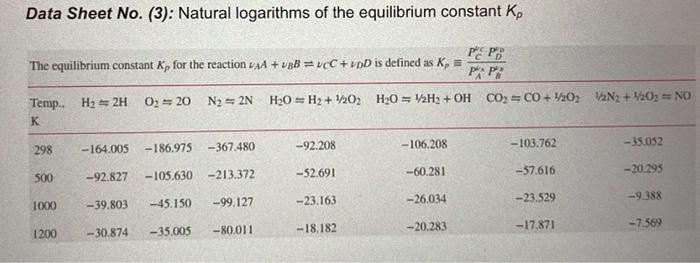
Liquid propane (C3H8) enters a combustion chamber at 25C at a rate of 1.2 kg/min where it is mixed and burned with 150 percent excess air that enters the combustion chamber at 12C. The combustion gases consist of CO2, H2O, CO, O2, and N2 that exit at 1200 K and 1.9 atm. a. Show a simplified PFD of the process and determine the theoretical combustion equation if complete combustion is assumed with no excess oxygen. [3 Marks] b. Determine the equilibrium composition of the product gases. (10 Marks] c. Determine the rate of heat transfer from the combustion chamber (h values are given). [10 Marks] d. Is it realistic to disregard the presence of NO in the product gases? Why? [2 marks] Data Supplied: Substance K C3H8 (1) O2 N2 H20 (9) CO2 h; kJ/kmol h285 k kJ/kmol h298 K kJ/kmol h 1200k kJ/kmol -118910 8296.5 8682 38447 0 8286.5 8669 36777 9904 44380 -241820 -393520 9364 53848 Data Sheet No (3) Natural logarithms of the equilibrium constant ko Data Sheet No. (3): Natural logarithms of the equilibrium constant Kp PEPO The equilibrium constant Ky for the reaction 1AA + B = VCC + vpD is defined as K, = 02 = 20 N2 = 2N H20 = H2 + 1/202H20 = 12H2 + OH CO2 =CO+ V2022N2 +120, NO Temp. H2 =2H K 298 - 164.005 -367.480 -103.762 -186.975 -92.208 - 35.052 -106.208 500 -92.827 -52.691 -60.281 - 57.616 -20.295 -105.630 -213.372 -39.803 -99.127 1000 -45.150 -23.163 --26.034 - 23.529 -9.388 1200 -35.005 -20.283 -7.569 -30.874 -80.011 -18.182 -17.871
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


