Question: Need Help with question 5-9. Consider an atom with 50 protons, 48 electrons, and 68 neutrons: Select every description below that would be an isotope
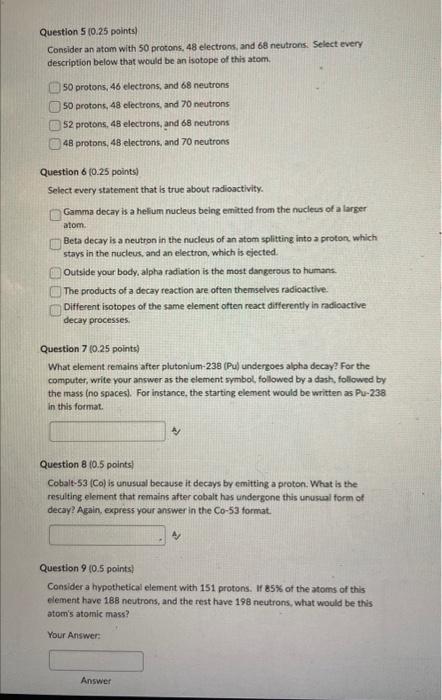
Consider an atom with 50 protons, 48 electrons, and 68 neutrons: Select every description below that would be an isotope of this atem. 50 protons, 46 electrons, and 68 neutrons 50 protons, 48 clectrons, and 70 neutrons. 52 protons, 48 electrons, and 68 neutrons 48 protons, 48 electrons, and 70 neutrons Question 6 (0.25 points) Select every statement that is true about radioactivity. Gamma decay is a helfum nucleus being emitted from the nucleus of a larger atom. Beta decay is a neutron in the nucleus of an atom splitting into a proton. which stays in the nucleus, and an electron, which is cjected. Outside your body, alpha radiation is the most dangerous to humans. The products of a decay reaction are often themselves radioactive. Different isotopes of the same element often react differently in madioactive decay processes. Question 7 (0.25 points) What element remains after plutonium-238 (Pu) undergoes alpha decay? For the computer, write your answor as the element symbol, followed by a dash, followed by the mass (no snaces). For instance, the starting element would be written as Pu-238 in this format. Question 8(0.5 points) Cobalt-53 (Co) is unusual because it decays by emitting a proton. What is the resulting element that remains after cobalt has undergone this unusual form of decay? Again. express your answer in the Co-53 format. Question 9 (0.5 points) Consider a hypothetical element with 151 protons. If 85% of the atoms of this element have 188 neutrons, and the rest have 198 neutrons, what would be this atom's atomic mass? Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


