Question: need help with red boxes Write the formula for the conjugate base of each of the following acids: (a) CH2ClCOOH (x) (b) HIO4 (c) H2SO4
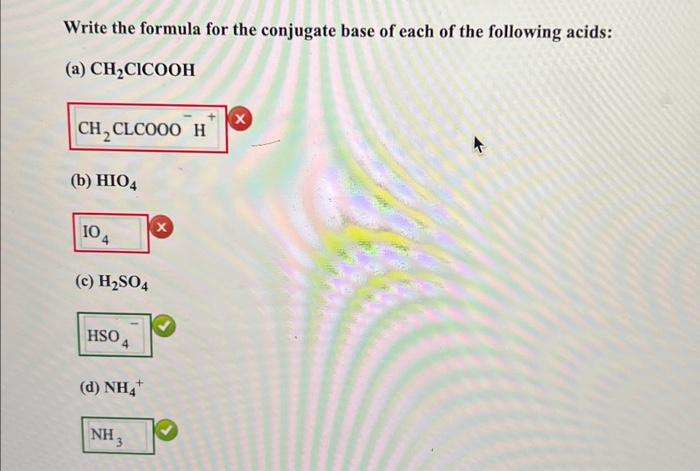
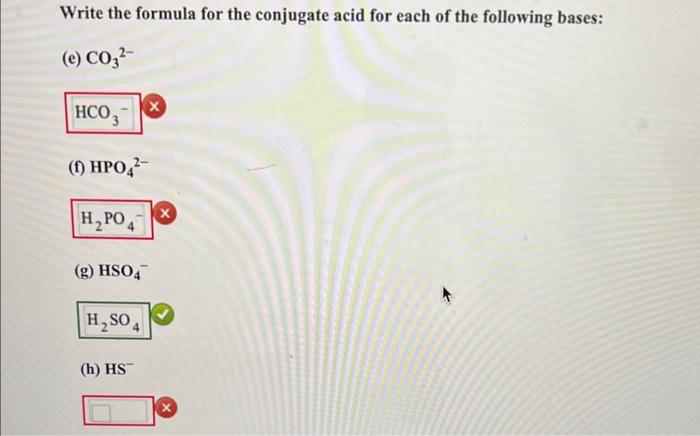
Write the formula for the conjugate base of each of the following acids: (a) CH2ClCOOH (x) (b) HIO4 (c) H2SO4 (d) NH4+ Write the formula for the conjugate acid for each of the following bases: (e) CO32 (f) HPO42 (g) HSO4 (h) HS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


