Question: need help with the blanks. please include calculations! thank you! Molar Mass Determination by Depression of the Freezing Point DATA Check all entries for consistent
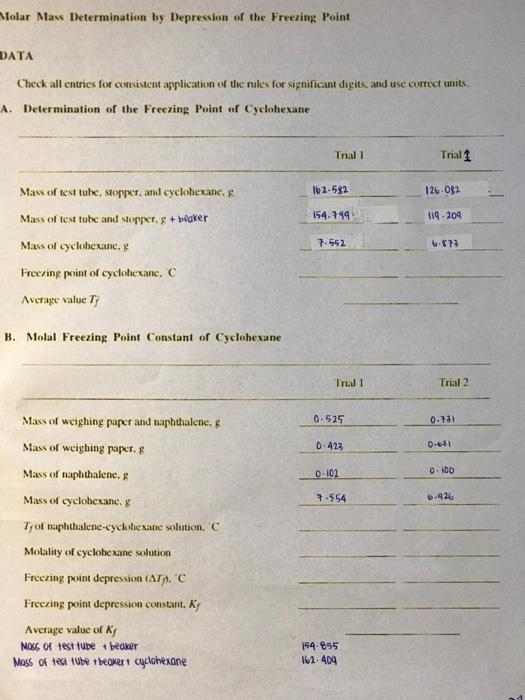

Molar Mass Determination by Depression of the Freezing Point DATA Check all entries for consistent application of the rules for significant digits, and use correct units A. Determination of the Freezing Point of Cyclohexane Trall Trial 1 162-582 126-082 154.799 119.204 Mass of test tube, stopper, and cyclohexane, 8 Mass of test tube and stopper. g + beaker Mass of cyclohexane. ! Freezing point of cyclohexanc. C Average value Ty 7.552 6.73 B. Molal Freezing Point Constant of Cyclohexane Tradi Trial 2 0.525 0.13 Mass of weighing paper and naphthalene, 8 Mass of weighing paper, Mass of naphthalene. 0.423 D-621 0.102 0-100 Mass of cyclohexane. & 7.554 6-426 Tror naphthalene-cyclohexane solution, C Molality of cyclohexane solution Freezing point depression (ATH.C Freezing point depression constant. Ky Average value of Kj Nos of test tube + beaker Mass of test tube + beover cyclohexane 154.655 162.404 Experimen Moler Sass Dein Report Name Section Molar Mass Determination by Depression of the Freezing Point C. The Molar Mass of an Unknown Solid Trial! Trial 2 Unknown Number 2 162.257 162.60 194-80% 154.839 7.554 7.769 0.645 0-$40 03. 0.138 Mass of test tube, stopper and cyclohexane. 8 Mass of test tube and stopper Mass of cyclohexane 8 Mass of weighing paper and unknown simple. g Mass of weighing paper, Mass of unknown samples Trof unknown-cyclohexane solution. C Freezing point depression (AT). C Molality of unknown-cyclohexane solution Morlar mass of unknown. g mok! Average value of molar mass, g mot! 0-109 0-102 Sample Calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


