Question: need help with the four I got wrong! thank you Determine the frequency and energy for light with a wavelength of 717.7nm. v= E: Vmol
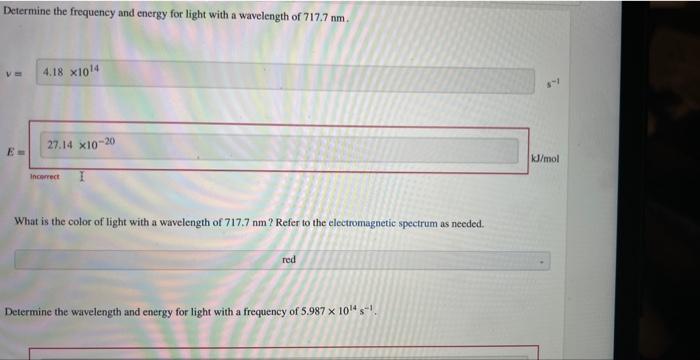
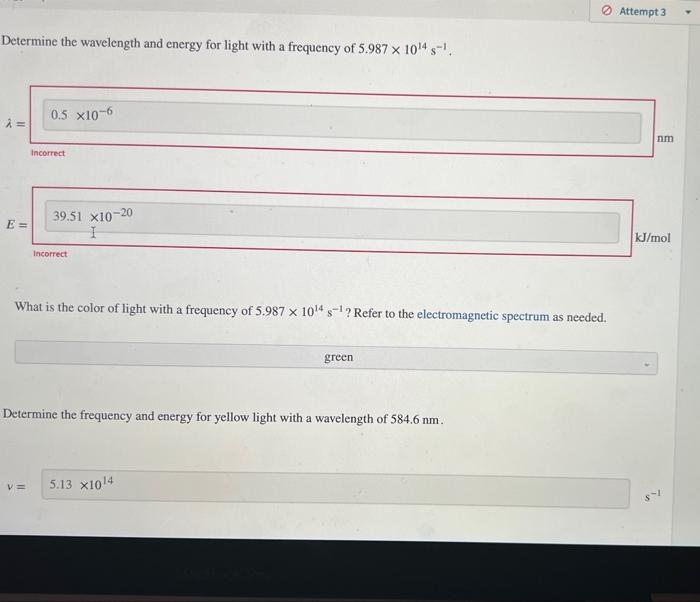
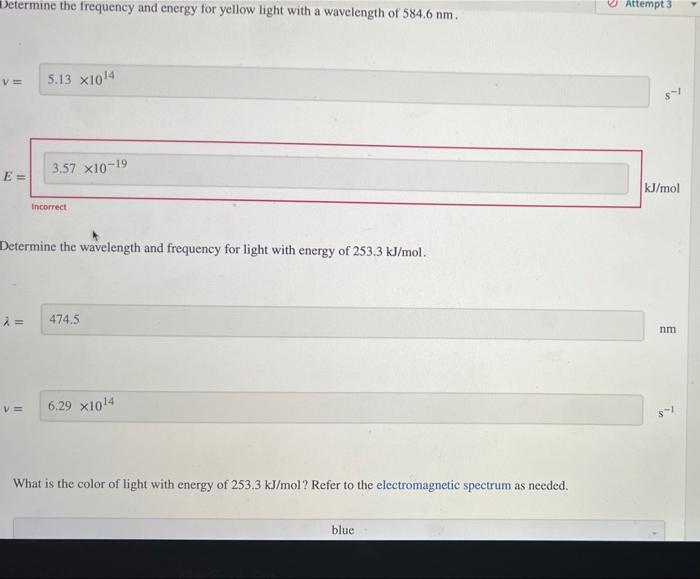
Determine the frequency and energy for light with a wavelength of 717.7nm. v= E: Vmol incerect 1 What is the color of light with a wavelength of 717.7nm ? Refer to the electromagnetic spectrum as needed. Determine the wavelength and energy for light with a frequency of 5.9871014s1. Determine the wavelength and energy for light with a frequency of 5.9871014s1. What is the color of light with a frequency of 5.9871014s1 ? Refer to the electromagnetic spectrum as needed. Determine the frequency and energy for yellow light with a wavelength of 584.6nm. Determine the frequency and energy for yellow light with a wavelength of 584.6nm. Determine the wavelength and frequency for light with energy of 253.3kJ/mol. = What is the color of light with energy of 253.3kJ/mol ? Refer to the electromagnetic spectrum as needed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


