Question: Need help with these 3 problems pls helps Compare the following: Acid 1: phosphoric acid, H3PO4 Acid 2: hydrogen sulfide ion, HS Acid 3: carbonic

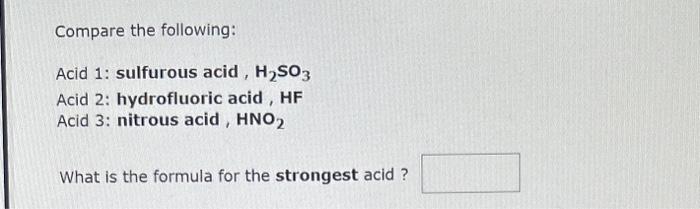
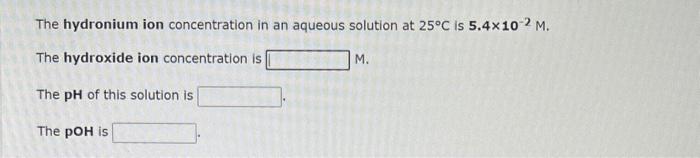
Compare the following: Acid 1: phosphoric acid, H3PO4 Acid 2: hydrogen sulfide ion, HS Acid 3: carbonic acid, H2CO3 What is the formula for the strongest acid? Compare the following: Acid 1: sulfurous acid, H2SO3 Acid 2: hydrofluoric acid, HF Acid 3: nitrous acid, HNO2 What is the formula for the strongest acid ? The hydronium ion concentration in an aqueous solution at 25C is 5.4102M. The hydroxide ion concentration is M. The pH of this solution is The pOH is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


