Question: need help with these problems The illustration to the left represents a mixture of nitrogen (blue ) and oxygen (red) molecules. If the molecules in

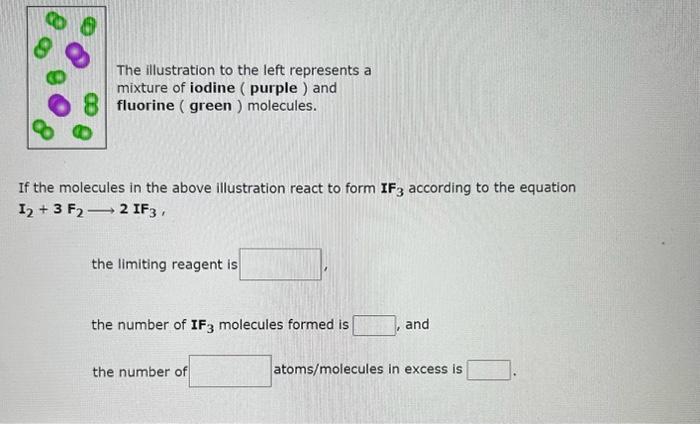
The illustration to the left represents a mixture of nitrogen (blue ) and oxygen (red) molecules. If the molecules in the above illustration react to form NO2 according to the equation N2+2O22NO2, the limiting reagent is the number of NO2 molecules formed is and the number of molecules in excess is The illustration to the left represents a mixture of iodine (purple) and fluorine (green ) molecules. If the molecules in the above illustration react to form IF3 according to the equation I2+3F22IF3, the limiting reagent is the number of IF3 molecules formed is and the number of atoms/molecules in excess is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


