Question: Need help with this please. need more help in b. 5) Consider the container below. The volume of the left side and the volume of
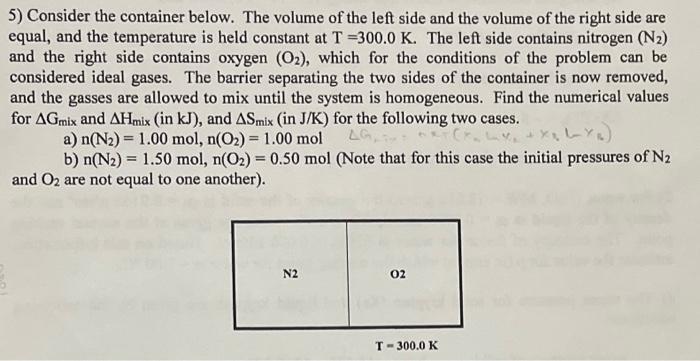
5) Consider the container below. The volume of the left side and the volume of the right side are equal, and the temperature is held constant at T=300.0K. The left side contains nitrogen (N2) and the right side contains oxygen (O2), which for the conditions of the problem can be considered ideal gases. The barrier separating the two sides of the container is now removed, and the gasses are allowed to mix until the system is homogeneous. Find the numerical values for Gmix and Hmix (in kJ ), and Smix (in J/K ) for the following two cases. a) n(N2)=1.00mol,n(O2)=1.00mol b) n(N2)=1.50mol,n(O2)=0.50mol (Note that for this case the initial pressures of N2 and O2 are not equal to one another)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


