Question: Need help with this question with full solution to the answer Question 1 (1 point) With respect to the information below, estimate the precision of
Need help with this question with full solution to the answer 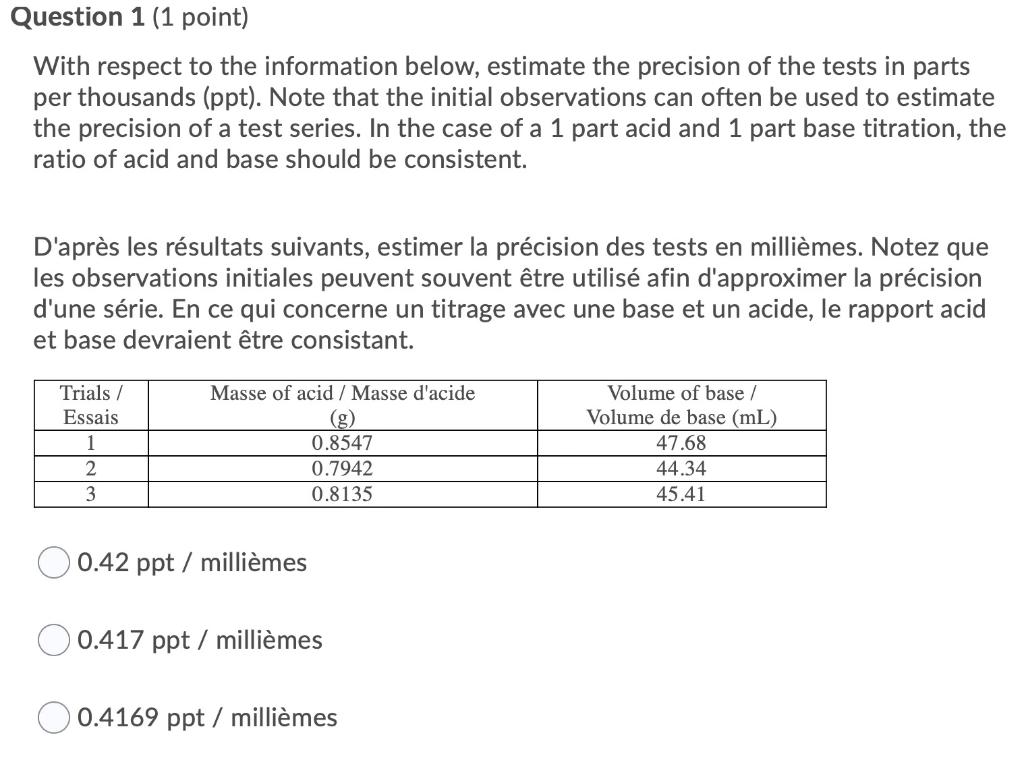
Question 1 (1 point) With respect to the information below, estimate the precision of the tests in parts per thousands (ppt). Note that the initial observations can often be used to estimate the precision of a test series. In the case of a 1 part acid and 1 part base titration, the ratio of acid and base should be consistent. D'aprs les rsultats suivants, estimer la prcision des tests en millimes. Notez que les observations initiales peuvent souvent tre utilis afin d'approximer la prcision d'une srie. En ce qui concerne un titrage avec une base et un acide, le rapport acid et base devraient tre consistant. Masse of acid / Masse d'acide Trials / Essais 1 2 3 0.8547 0.7942 0.8135 Volume of base / Volume de base (mL) 47.68 44.34 45.41 0.42 ppt / millimes 0.417 ppt / millimes 0.4169 ppt / millimes Question 1 (1 point) With respect to the information below, estimate the precision of the tests in parts per thousands (ppt). Note that the initial observations can often be used to estimate the precision of a test series. In the case of a 1 part acid and 1 part base titration, the ratio of acid and base should be consistent. D'aprs les rsultats suivants, estimer la prcision des tests en millimes. Notez que les observations initiales peuvent souvent tre utilis afin d'approximer la prcision d'une srie. En ce qui concerne un titrage avec une base et un acide, le rapport acid et base devraient tre consistant. Masse of acid / Masse d'acide Trials / Essais 1 2 3 0.8547 0.7942 0.8135 Volume of base / Volume de base (mL) 47.68 44.34 45.41 0.42 ppt / millimes 0.417 ppt / millimes 0.4169 ppt / millimes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


