Question: Need only typed answer by keyboard Solve fast (A 33% Part (a) What pressure (in kPa) is the helium gas under? [A 33% Part (b)
Need only typed answer by keyboard
Solve fast
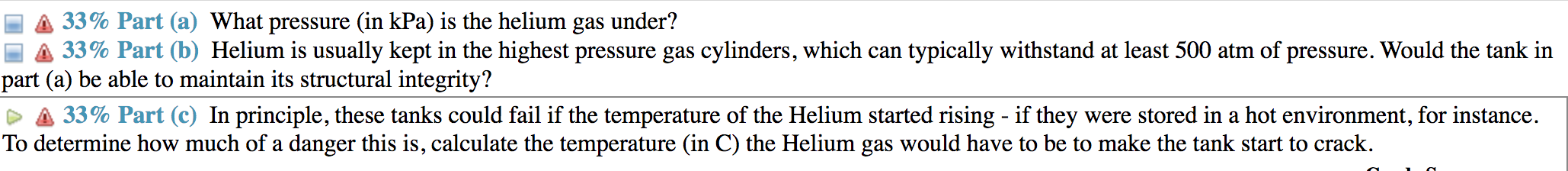
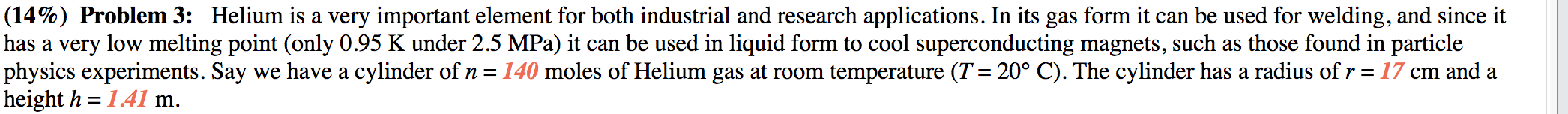
(A 33% Part (a) What pressure (in kPa) is the helium gas under? [A 33% Part (b) Helium is usually kept in the highest pressure gas cylinders, which can typically Withstand at least 500 atm of pressure. Would the tank in part (a) be able to maintain its structural integrity? & 33% Part (c) In principle, these tanks could fail if the temperature of the Helium started rising - if they were stored in a hot environment, for instance. To determine how much of a danger this is, calculate the temperature (in C) the Helium gas would have to be to make the tank start to crack. (14%) Problem 3: Helium is a very important element for both industrial and research applications. In its gas form it can be used for welding, and since it has a very low melting point (only 0.95 K under 2.5 MPa) it can be used in liquid form to cool superconducting magnets, such as those found in particle physics experiments. Say we have a cylinder of n = 140 moles of Helium gas at room temperature (T: 20 C). The cylinder has a radius of r = 17 cm and a height h = 1.41 m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


