Question: Need part b) thank you! 1. a. A ruby crystal has a composition (Alo.99Cr0.01)203. How many Cr3+ ions are there in a ruby of dimensions
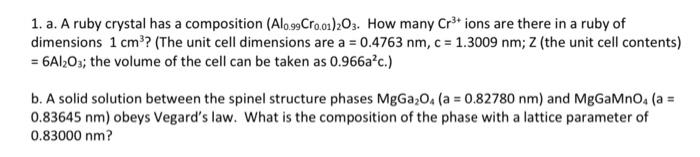
1. a. A ruby crystal has a composition (Alo.99Cr0.01)203. How many Cr3+ ions are there in a ruby of dimensions 1 cm? (The unit cell dimensions are a = 0.4763 nm, c = 1.3009 nm; 2 (the unit cell contents) = 6A1203; the volume of the cell can be taken as 0.966ac.) b. A solid solution between the spinel structure phases MgGa0(a = 0.82780 nm) and MgGaMno. (a = 0.83645 nm) obeys Vegard's law. What is the composition of the phase with a lattice parameter 0.83000 nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


