Question: Need to make graph, please answer Question a-f. Will rate, thanks. Question 1: Construct the calibration curve (60%) a- Obtain the average area of methanol
Need to make graph, please answer Question a-f. Will rate, thanks.
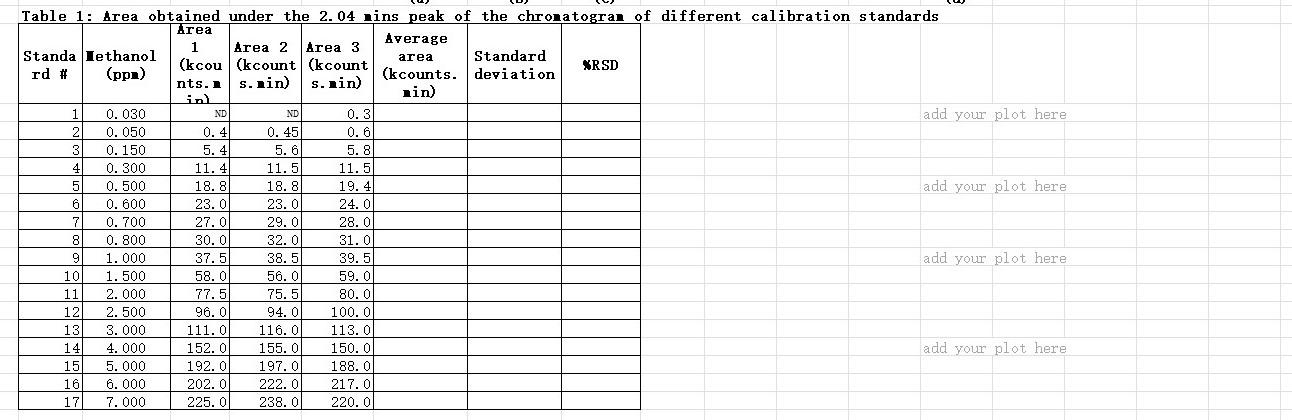
Question 1: Construct the calibration curve (60%) a- Obtain the average area of methanol peak of each standard provided in the excel sheet (consider that there are no outliers) b- Obtain the standard deviation of the area of methanol of each standard provided in the excel sheet C- Obtain the %RSD of each standard analyzed d- Plot the calibration curve (taking the average area as the signal) with all values provided except for standards #1 (0.030 ppm) and #2 (0.050 ppm). (Provide title, axls titles, and appropriate units) e Obtain the "initial equation" of the straight line and "initial R2", and comment if this equation is suitable for quantification. f- Remove minimum amount from the higher concentration standards to make the line linear (try to get it to 0.999). Give the final equation of the straight line and R2 obtained. Table 1: Area obtained under the 2.04 nins peak of the chronatograi of different calibration standards Area Average 1 Standa Iethanol Area 2 Area 3 area (kcou (kcount (kcount Standard rd # (ppa) XRSD (kcounts. deviation nts. s.nin) s.ain) in rin) 0.030 ND ND 0.3 add your plot here 2 0.050 0.4 0.45 0.6 3 0.150 5.4 5. 6 5.8 4 0.300 11.4 11.5 11.5 5 0.500 18.8 18.8 19.4 add your plot here 6 0.600 23. O 23.0 24.0 7 0.700 27.0 29.0 28.0 8 0.800 30.0 32.0 31.0 9 1.000 37.5 38.5 39.5 add your plot here 10 1.500 58.0 56. O 59.00 11 2.000 77.5 75.5 80.0 12 2. 500 96.0 94. O 100.0 13 3.000 111.0 116.0 113.0 14 4. 000 152.0 155. O 150.0 add your plot here 15 5. 000 192.0 197.0 188.0 16 6.000 202.0 222.0 217.0 17 7.000 225.01 238.0 220.01
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


