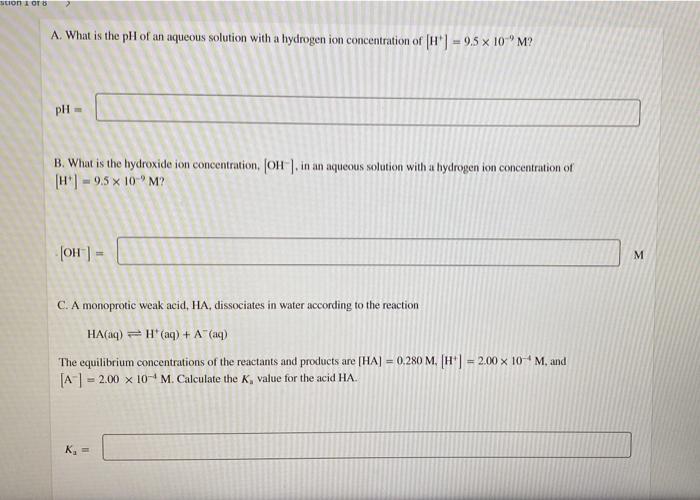
Question: Need urgent help!!! son OT A. What is the pH of an aqueous solution with a hydrogen ion concentration of H] = 9.5 x 10
![aqueous solution with a hydrogen ion concentration of H"] = 9.5 x](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9424da30fd_46966f9424d43c18.jpg)
son OT A. What is the pH of an aqueous solution with a hydrogen ion concentration of H"] = 9.5 x 10 "M? pH B. What is the hydroxide ion concentration. (OH) in an aqueous solution with a hydrogen ion concentration of H] 9.5 10-"M? JOH- M C. A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H(aq) + A" (aq) The equilibrium concentrations of the reactants and products are [HA] = 0.280 M. (H"] = 2.00 x 10-M, and [A-1 = 2.00 x 10- M. Calculate the K, value for the acid HA. K = A monoprotic weak acid, HA. dissociates in water according to the reaction HA(n)H(aq) + (aq) The equilibrium concentrations of the reactants and products are [HA] = 0.220 M. (H) = 4.00 x 10-M, and [A-] = 4,00 x 10-6 M. Calculate the value of pK, for the acid HA. pk =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


