Question: need zero order, first order, and second order graphs with R^2 values for all of them Graphically determine the rate order given the data below

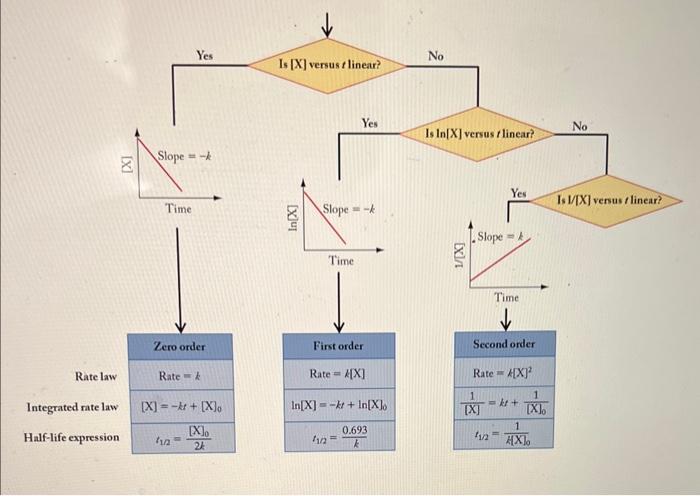
Graphically determine the rate order given the data below for A+(aq)+D(s)=B2+(aq)+s solid by products. Time (s)[A+1 02350 602.252 1202144 1802.044 2401942 3001.840 3601738 Is ln[X] versus t linear? No
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


