Question: nit for sure what i did wrong in my equation Microwave ovens work by irradiating food with microwave radiation, which is absorbed and converted into
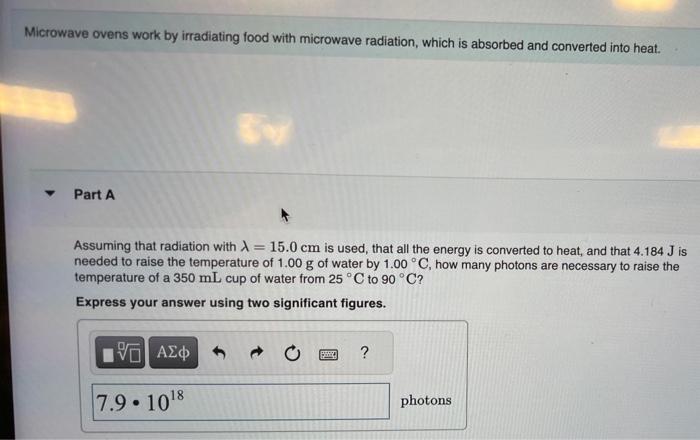
Microwave ovens work by irradiating food with microwave radiation, which is absorbed and converted into heat. Part A Assuming that radiation with =15.0cm is used, that all the energy is converted to heat, and that 4.184J is needed to raise the temperature of 1.00g of water by 1.00C, how many photons are necessary to raise the temperature of a 350mL cup of water from 25C to 90C ? Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


