Question: Nitrate Reduction Test Anaerobic Respiration - Nitrate Reduction Test Objectives: After completing this lab you should be able to: - Use aseptic technique to properly


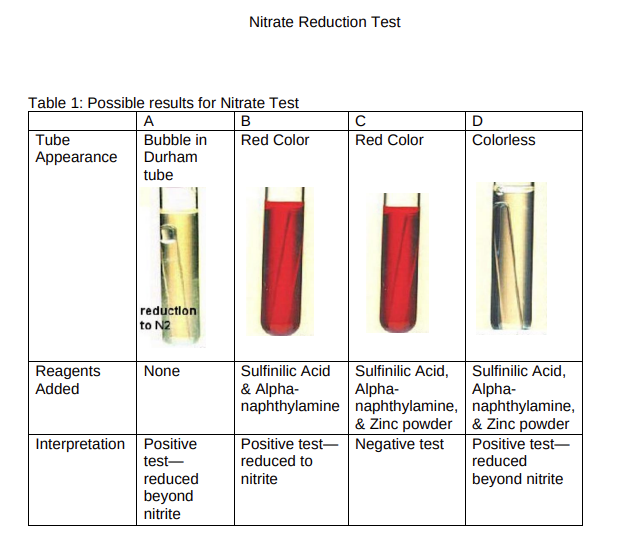

Nitrate Reduction Test Anaerobic Respiration - Nitrate Reduction Test Objectives: After completing this lab you should be able to: - Use aseptic technique to properly inoculate a nitrate broth tube - Interpret the results of nitrate broth tubes, including the symbiotic, cooperative reduction of nitrate to nitrogen gas by a mixed culture of 2 species. Introduction: Anaerobic respiration is the metabolic process in which an inorganic molecule other than oxygen serves as the final electron acceptor in the electron transport chain. Nitrate reduction is one form of anaerobic respiration. Many bacteria have the enzyme nitrate reductase which can reduce nitrate (NO3)to nitrite (NO2)and some can reduce it even further, even as far as N2 (nitrogen gas), using additional enzymes (Figure 1). The nitrate test is used to detect the ability of a microorganism to reduce nitrate to nitrite, or to a nitrogenous compound "beyond nitrite." Figure 1: Possible end products of nitrate reduction. In the nitrate test, the bacteria are grown in a broth medium that contains potassium nitrate in addition to other components required for growth. An inverted Durham tube is placed in the culture broth to collect gas produced during the incubation period. If no gas is produced, a series of chemicals (reagents) are added after incubation during the interpretation of results. Inoculation: 1. Label the tube only with the initials of the bacterial species. 2. Check that the Durham tube is filled with liquid before inoculation. If any gas bubble is present in the Durham tube, gently invert the nitrate broth tube to allow the gas to escape the Durham tube. 3. Three of the tubes will be inoculated with only one species each (Escherichia coli, Serratia marcescens, or Alcaligenes faecalis). 4. Inoculate the fourth tube with both E.coli and Alcaligenes faecalis (one at a time, flame loop in between the transfers!). This will demonstrate the symbiotic effort of these two species being able to do what neither can do alone. 5. After inoculation, place the tubes in the appropriate rack on the side bench for incubation. Interpretation of Nitrate Test Results: 1. After incubation, the first step to check the Durham tube for the presence of a gas bubble. If gas is present, this indicates that the bacteria reduced the nitrate in the tube 'beyond nitrite' to form molecular nitrogen and/or nitrous oxide gas. In this case, no further testing is done (see Table 1-A). 2. If no gas is present in the Durham tube, 7-8 drops each of sulfinilic acid and alphanaphthylamine are added to the tube. If nitrite (NO2) is present, these two chemicals react with the nitrite to produce a red, water soluble compound. The development of a red color in the tube within 15 minutes of adding these chemicals indicates that the bacteria reduced the nitrate in the tube to nitrite (Table 1-B). Note that the red color may fade to a rusty orange color over time in the demonstration tubes. 3. If the tube did not turn red after adding sulfinilic acid and alpha-naphthylamine (and there was no gas bubble in the Durham tube), there are two possible results to consider: The bacteria did not reduce the nitrate in the tube OR the bacteria reduced the nitrate beyond nitrite to produce ammonia (which is not a gas). To distinguish between these two results, a small amount of powdered zinc is added to the tube. Zinc catalyzes the reduction of nitrate into nitrite. If the bacteria did not reduce the nitrate, the zinc will convert the nitrate in the tube into nitrite. Because sulfinilic acid and alphanaphthylamine were added in the previous step, a red color will start to form in the tube as nitrite is produced by the zinc (Table 1-C). Note that the red color may fade to a rusty orange color over time in the demonstration tubes. If the bacteria reduced the nitrate to ammonia, there will not be a color change in the tube. There is no nitrate left for the zinc to turn into nitrite (and no red color will be produced by sulfinilic acid and alphanaphthylamine) (Table 1-D). Nitrate Reduction Test Use the rack of demonstration tubes and the "Reagents Added" table attached to the rack to fill-in the "Results" table below. Please be gentle when handling the nitrate tubes so as not to disturb any gas bubbles
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


