Question: Nitrogen atom (-N) has high electron affinity than carbon atom (C). Select one: O True O False Question 10 Given: C(s) + O2(g) -


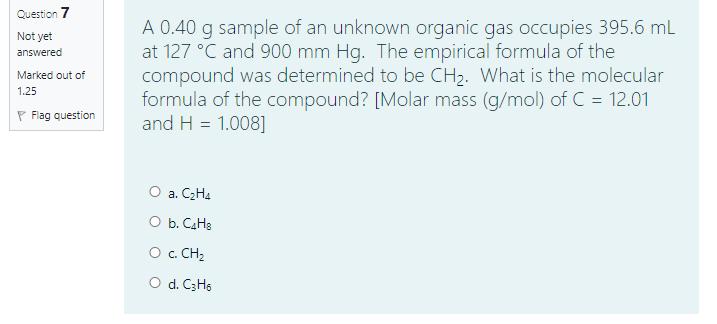
Nitrogen atom (-N) has high electron affinity than carbon atom (C). Select one: O True O False Question 10 Given: C(s) + O2(g) - CO2(g) AH pn = -393.5 kJ. What is the enthalpy change (AH pyn) for rxn Not yet the following reaction? 2C(s) + 20;(g) - 2CO,(g) answered Marked out of 1.25 P Flag question O a. -393.5 kJ O b. 787 kJ O c.- 787 kJ O d. 196.75 kJ Question 7 A 0.40 g sample of an unknown organic gas occupies 395.6 mL at 127 C and 900 mm Hg. The empirical formula of the compound was determined to be CH2. What is the molecular formula of the compound? [Molar mass (g/mol) of C = 12.01 and H = 1.008] Not yet answered Marked out of 1.25 P Flag question O a. C2H4 O b. CaHs O . CH2 O d. C3H6
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


