Question: Nitrogen expands through a nozzle from a negligible initial velocity to a final velocity of 3 2 5 m s . Assuming nitrogen an ideal
Nitrogen expands through a nozzle from a negligible initial velocity to a final velocity of
Assuming nitrogen an ideal gas for which what is the temperature drop of the gas? Pay
attention to the units of the quantities used in the calculation.
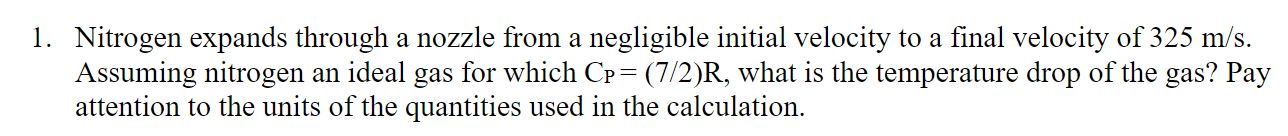
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


