Question: Nitrogen (N2) is heated as it flows through a pipe is heated from There is no pressure change as the N2 flows through the
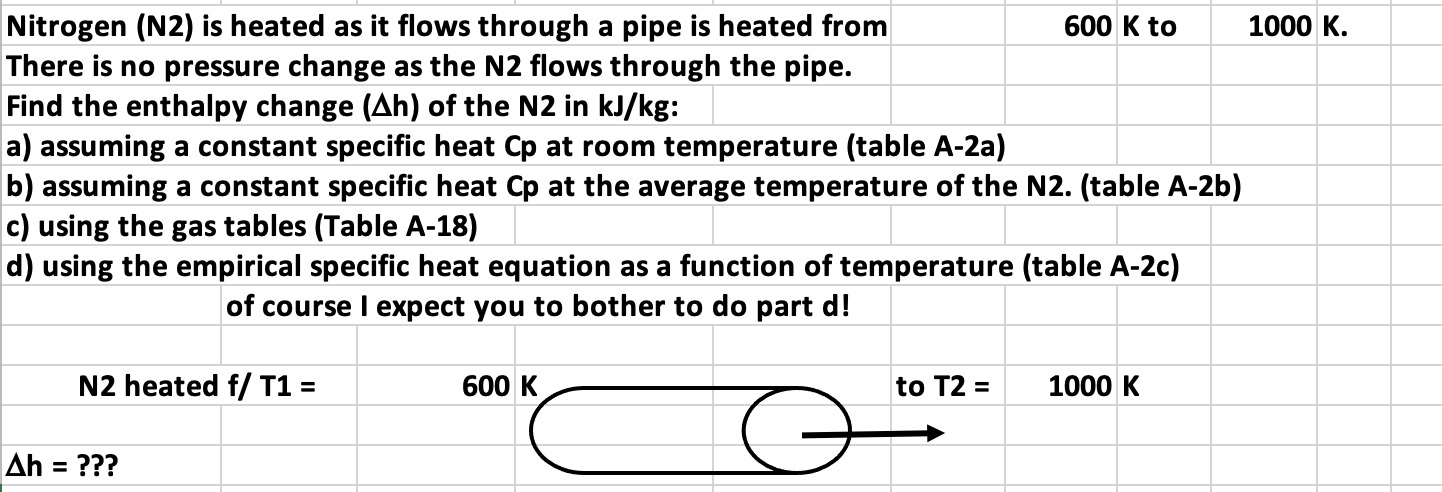
Nitrogen (N2) is heated as it flows through a pipe is heated from There is no pressure change as the N2 flows through the pipe. Find the enthalpy change (Ah) of the N2 in kJ/kg: a) assuming a constant specific heat Cp at room temperature (table A-2a) b) assuming a constant specific heat Cp at the average temperature of the N2. (table A-2b) c) using the gas tables (Table A-18) d) using the empirical specific heat equation as a function of temperature (table A-2c) of course I expect you to bother to do part d! N2 heated f/ T1 = Ah = ??? 600 K to 600 K to T2 = 1000 K 1000 K.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


