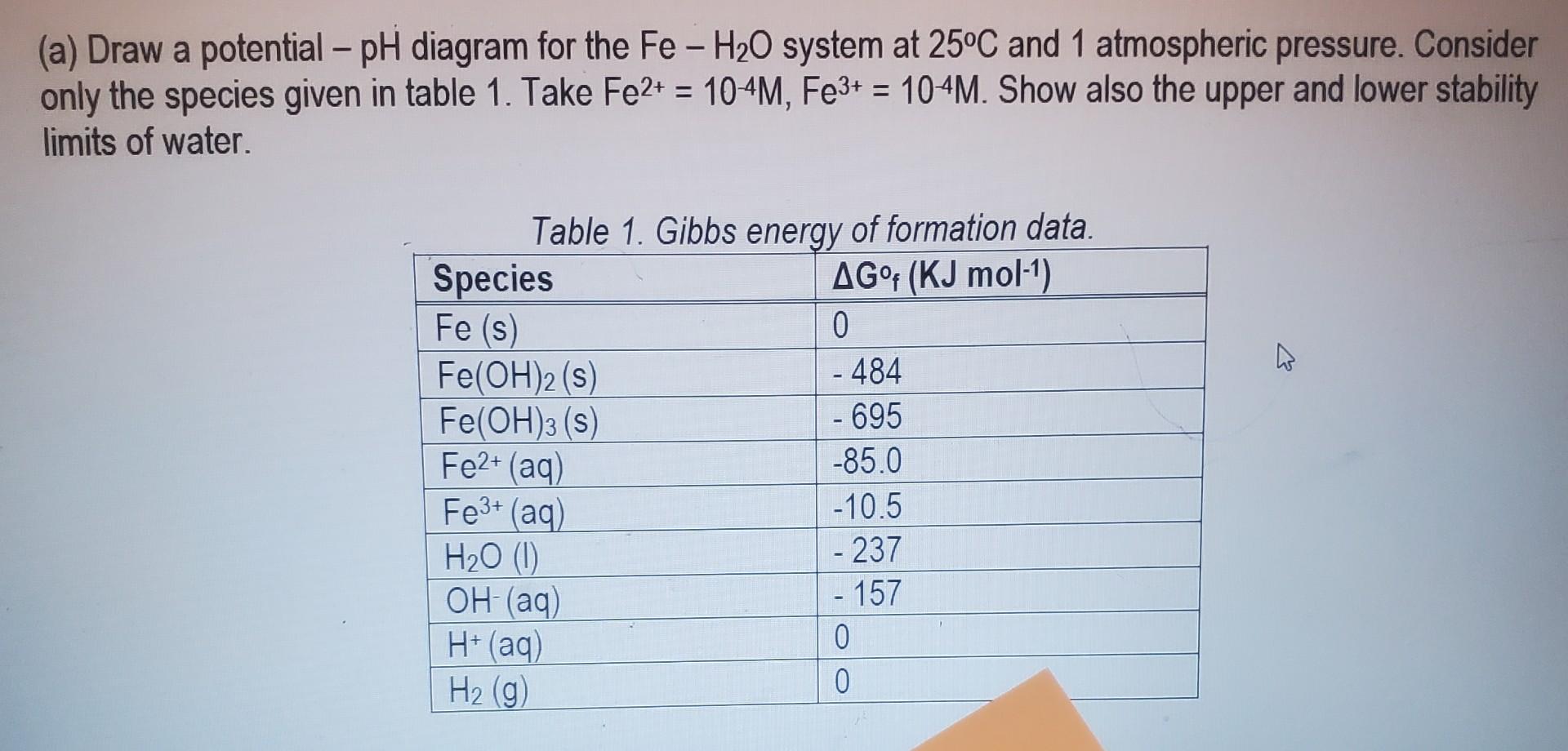
Question: no extra information had been given (a) Draw a potential - pH diagram for the FeH2O system at 25C and 1 atmospheric pressure. Consider only

no extra information had been given
(a) Draw a potential - pH diagram for the FeH2O system at 25C and 1 atmospheric pressure. Consider only the species given in table 1. Take Fe2+=104M,Fe3+=104M. Show also the upper and lower stability limits of water. Tahlo 1 Gihhe onarni of fnrmation data (b) Use your diagram to answer the following questions: (i) Is Fe stable in the presence of water at any pH values? (ii) What is the lowest pH value at which Fe(OH)3 is stable in the presence of Fe3+(aq) at a concentration of 104 mole/litre? (iii) What is the lowest pH value at which Fe(OH)2 is stable in the presence of Fe2+(aq) at a concentration of 104 mole/litre? (iv) If the pH were lowered, what would actually happen to Fe(OH)3 ? (v) Considering the redox couple Fe2+/Fe3+ on your FeH2O system, predict the behavior of dissolved iron at (i) anode (ii) cathode of an electrolytic system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


