Question: NO NEED SOLUTION. ANSWER ONLY THANK YOU Question 28 1 pts Given the thermochemical equation: 2N205 (g) 4NO2(g) + O2(g) AH = 110 kJ/mol How
NO NEED SOLUTION. ANSWER ONLY THANK YOU
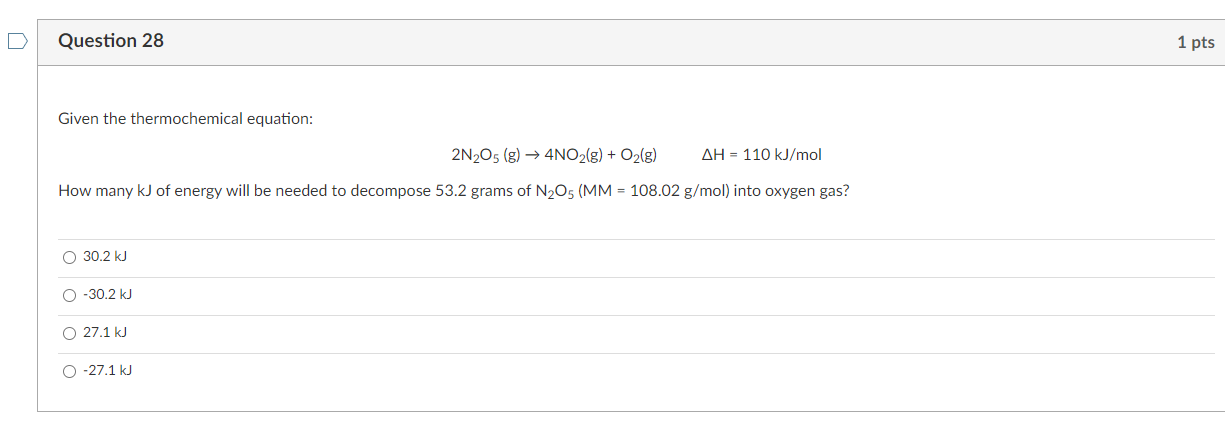
Question 28 1 pts Given the thermochemical equation: 2N205 (g) 4NO2(g) + O2(g) AH = 110 kJ/mol How many kJ of energy will be needed to decompose 53.2 grams of N2O5 (MM = 108.02 g/mol) into oxygen gas? O 30.2 kJ 0 -30.2 kJ O 27.1 kJ 0 -27.1 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


