Question: Not sure what step I got wrong What is the molecular formula of a compound with 62.04% carbon, 10.41% hydrogen, and 27.55% oxygen and a
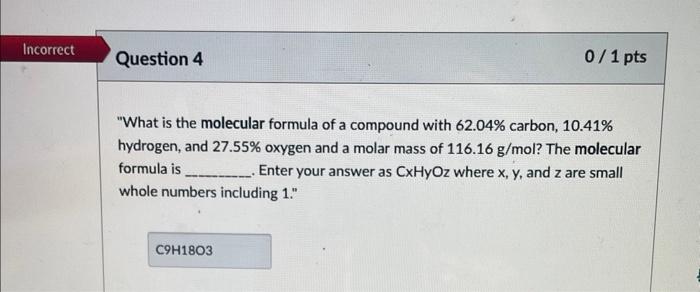

"What is the molecular formula of a compound with 62.04% carbon, 10.41% hydrogen, and 27.55% oxygen and a molar mass of 116.16g/mol ? The molecular formula is Enter your answer as CxHyOz where x,y, and z are small whole numbers including 1." C=762.04g12.01x1mul=5.166 H=710.41g1.008x1moll=1 0=727.54g16g1mol=1.121 1.721C5.166H10.3271.7210.7211.72127 C3H6O n=12(3)+6(6)+1(16)=88
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


