Question: Not sure what steps take place here- please help! Determining lonic Strength 12 of 18 Part A Review I Constants I Pyridine is a weak
Not sure what steps take place here- please help!
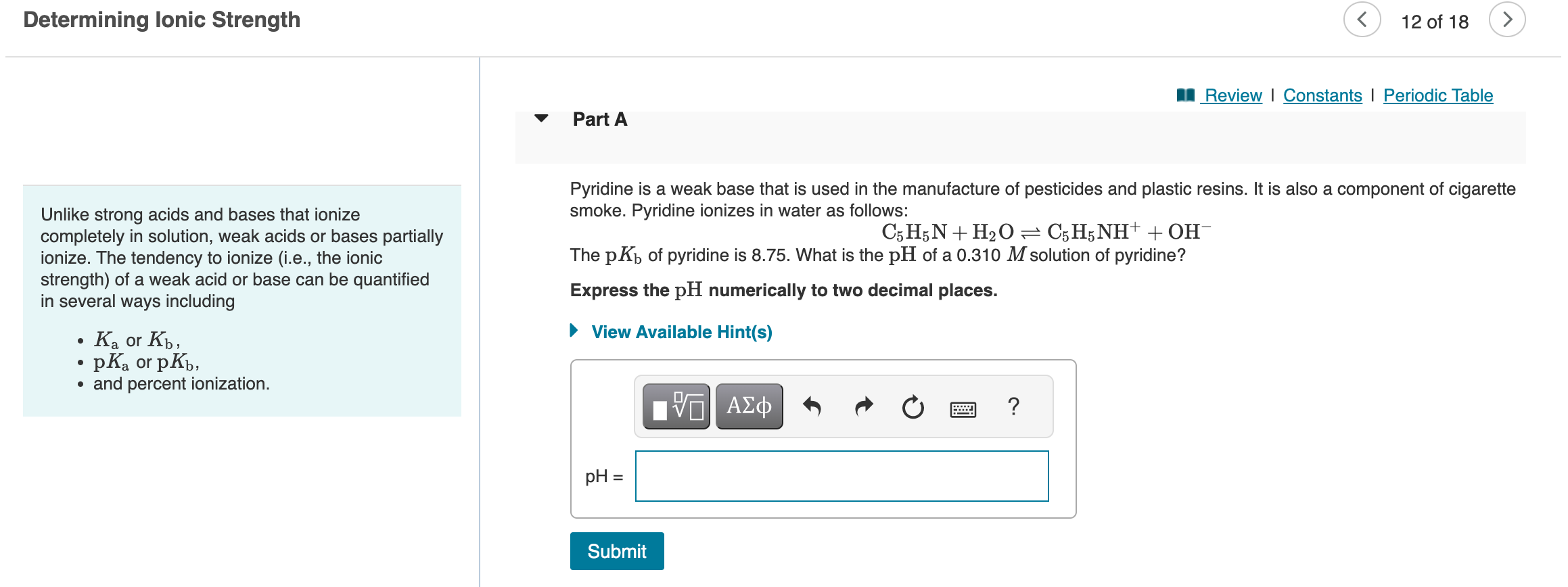
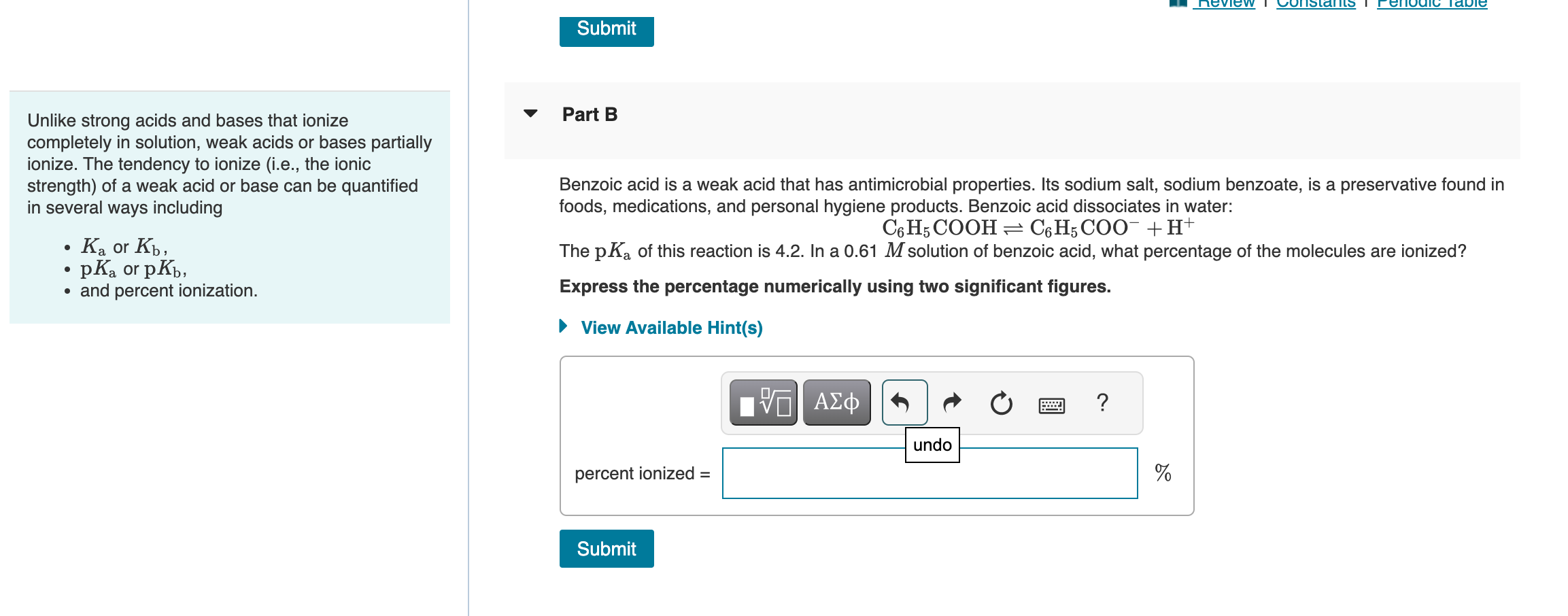
Determining lonic Strength 12 of 18 Part A Review I Constants I Pyridine is a weak base that is used in the manufacture of pesticides and plastic resins. It is also a component of cigarette Unlike strong acids and bases that ionize smoke. Pyridine ionizes in water as follows: completely in solution, weak acids or bases partially C5H5N+H2OC5H5NH++OH ionize. The tendency to ionize (i.e., the ionic The pKb of pyridine is 8.75. What is the pH of a 0.310M solution of pyridine? strength) of a weak acid or base can be quantified in several ways including Express the pH numerically to two decimal places. - Ka or Kb, - pKa or pKb, - and percent ionization. Unlike strong acids and bases that ionize Part B completely in solution, weak acids or bases partially ionize. The tendency to ionize (i.e., the ionic strength) of a weak acid or base can be quantified Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in in several ways including foods, medications, and personal hygiene products. Benzoic acid dissociates in water: - Ka or Kb, C6H5COOHC6H5COO+H+ - pKa or pKb, The pKa of this reaction is 4.2. In a 0.61M solution of benzoic acid, what percentage of the molecules are ionized? - and percent ionization. Express the percentage numerically using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


