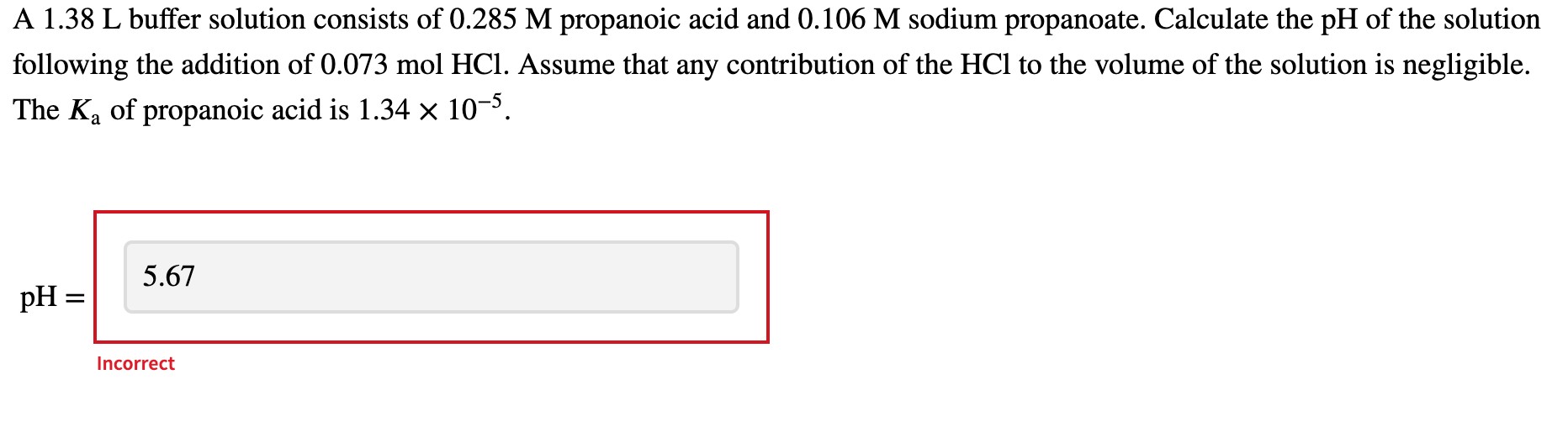
Question: Not sure why I got these incorrect A 1.38 L buffer solution consists of 0.285 M propanoic acid and 0.106 M sodium propanoate. Calculate the
 Not sure why I got these incorrect
Not sure why I got these incorrect
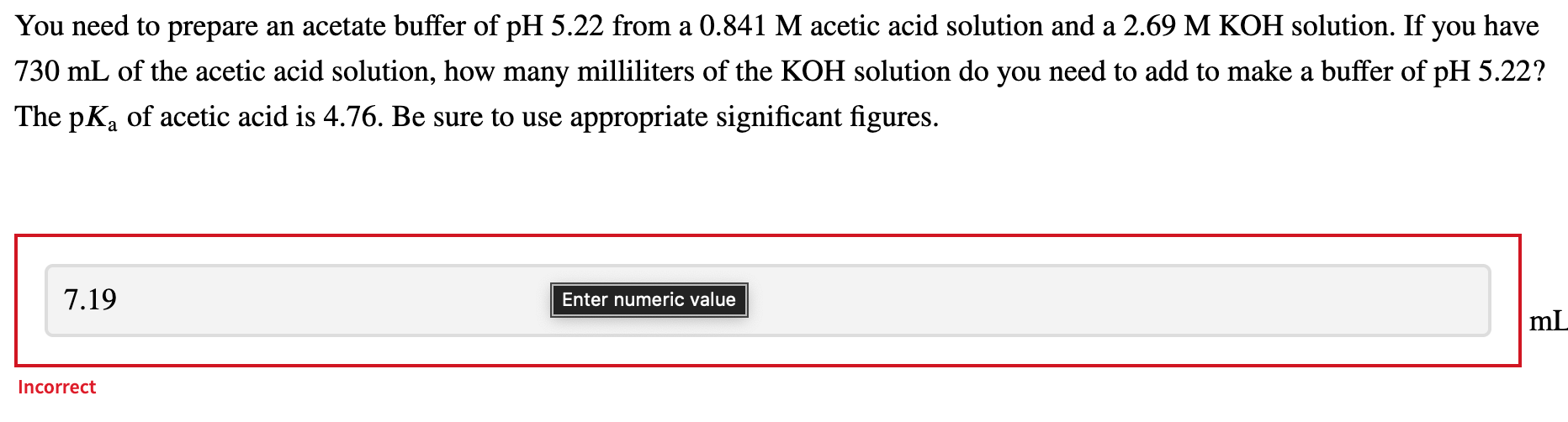
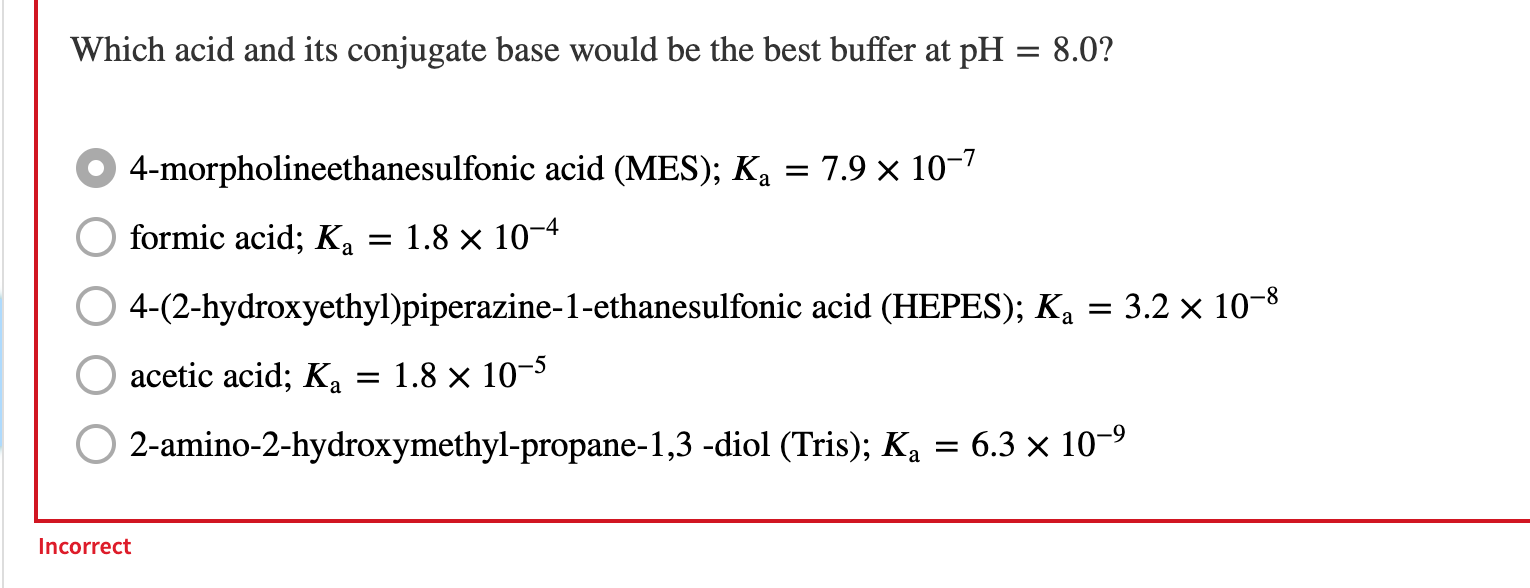
A 1.38 L buffer solution consists of 0.285 M propanoic acid and 0.106 M sodium propanoate. Calculate the pH of the solution following the addition of 0.073 mol HCl. Assume that any contribution of the HCl to the volume of the solution is negligible. The Ka of propanoic acid is 1.34 x 10-5. 5.67 pH = Incorrect a You need to prepare an acetate buffer of pH 5.22 from a 0.841 M acetic acid solution and a 2.69 M KOH solution. If you have 730 mL of the acetic acid solution, how many milliliters of the KOH solution do you need to add to make a buffer of pH 5.22? The pK, of acetic acid is 4.76. Be sure to use appropriate significant figures. a 7.19 Enter numeric value ml. Incorrect Which acid and its conjugate base would be the best buffer at pH = 8.0? = 4-morpholineethanesulfonic acid (MES); K = 7.9 x 10-7 = formic acid; Ka = 1.8 x 10-4 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES); Ka = 3.2 x 10-8 acetic acid; Ka = 1.8 x 10-5 2-amino-2-hydroxymethyl-propane-1,3 -diol (Tris); K4 = 6.3 x 10-9 Ka Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


