Question: Note that the single-pass conversion of hydrogen is 60.0%. Please solve for all the variables and calculate the overall conversion of hydrogen in the process
Note that the single-pass conversion of hydrogen is 60.0%. Please solve for all the variables and calculate the overall conversion of hydrogen in the process 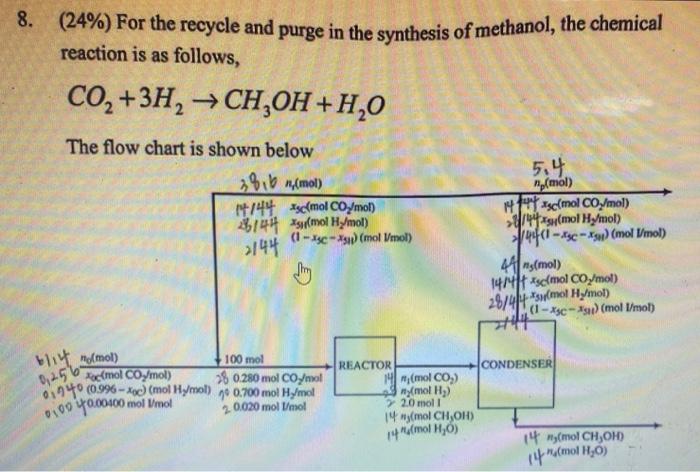

(24\%) For the recycle and purge in the synthesis of methanol, the chemical reaction is as follows, CO2+3H2CH3OH+H2O The flow chart is shown below
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


