Question: Note: there is NO final temperature!!!! please read the prompt, it is not the same as other questions posted here on this site!!! Set up
Note: there is NO final temperature!!!! please read the prompt, it is not the same as other questions posted here on this site!!!
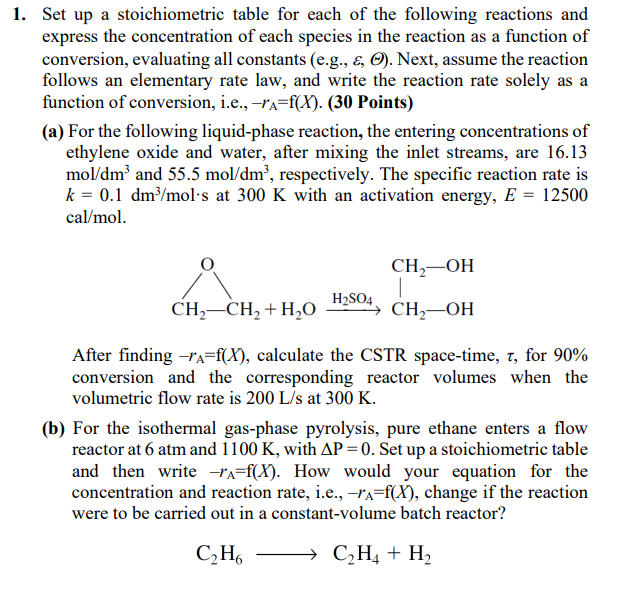
Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction as a function of conversion, evaluating all constants (e.g., , ). Next, assume the reaction follows an elementary rate law, and write the reaction rate solely as a function of conversion, i.e., rA=f(X). (30 Points) (a) For the following liquid-phase reaction, the entering concentrations of ethylene oxide and water, after mixing the inlet streams, are 16.13 mol/dm3 and 55.5mol/dm3, respectively. The specific reaction rate is k=0.1dm3/mols at 300K with an activation energy, E=12500 cal/mol. After finding rA=f(X), calculate the CSTR space-time, , for 90% conversion and the corresponding reactor volumes when the volumetric flow rate is 200L/s at 300K. (b) For the isothermal gas-phase pyrolysis, pure ethane enters a flow reactor at 6atm and 1100K, with P=0. Set up a stoichiometric table and then write rA=f(X). How would your equation for the concentration and reaction rate, i.e., rA=f(X), change if the reaction were to be carried out in a constant-volume batch reactor? C2H6C2H4+H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


