Question: Note: using Microsoft excel for solution Computer applications in Chemical Engineering HW # 1 A solution of 0.10 M nitric acid (HNO3) is saturated with
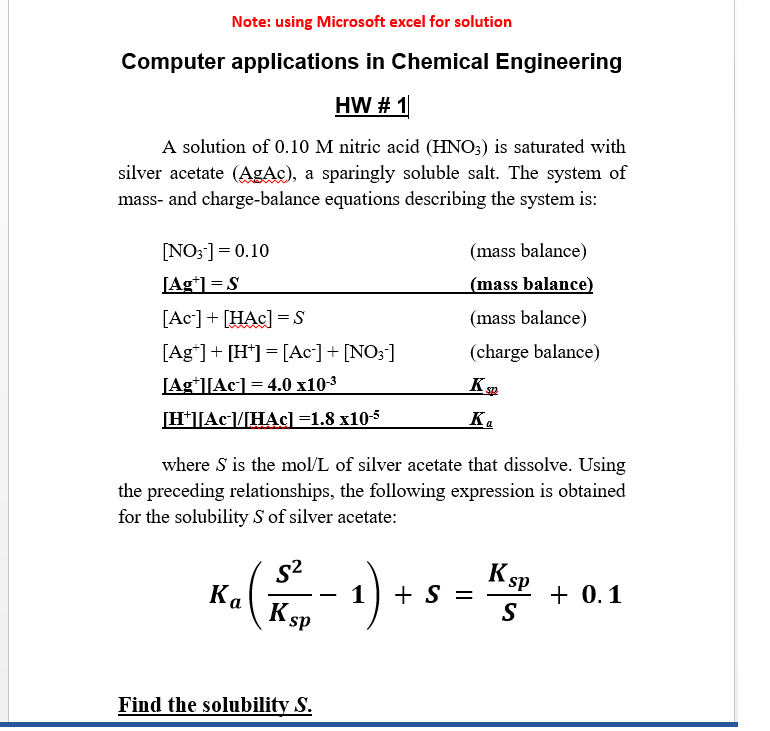
Note: using Microsoft excel for solution Computer applications in Chemical Engineering HW # 1 A solution of 0.10 M nitric acid (HNO3) is saturated with silver acetate (AgAc), a sparingly soluble salt. The system of mass- and charge-balance equations describing the system is: [NO3-] = 0.10 [Agt1 = S [Ac-] + [HAC] =S [Ag] + [H+] =[Ac-] + [NO3 ] [Ag||Ac] = 4.0 x10-3 [H+][Ac-/HAC) =1.8 x10-5 (mass balance) (mass balance) (mass balance) (charge balance) KS sp K. where S is the mol/L of silver acetate that dissolve. Using the preceding relationships, the following expression is obtained for the solubility S of silver acetate: S2 K SP Ka ( - 1) +- + S = K SP + 0.1 S Find the solubility S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


