Question: Now we apply the diffusion equation to Fig. 1. (a) Draw the shape for C(z) vs I just after partition removal (i.e. the gas totally
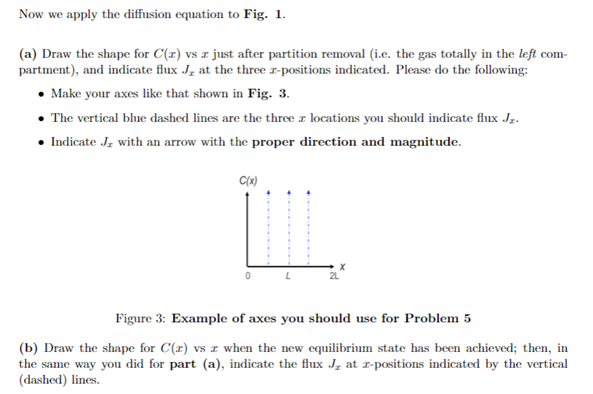
Now we apply the diffusion equation to Fig. 1. (a) Draw the shape for C(z) vs I just after partition removal (i.e. the gas totally in the left com- partment), and indicate flux J, at the three 2-positions indicated. Please do the following: Make your axes like that shown in Fig. 3. The vertical blue dashed lines are the three : locations you should indicate flux Jz. Indicate J, with an arrow with the proper direction and magnitude. C(x) 0 Figure 3: Example of axes you should use for Problem 5 (b) Draw the shape for C() vs I when the new equilibrium state has been achieved; then, in the same way you did for part (a), indicate the flux J, at 2-positions indicated by the vertical (dashed) lines. VS Now we apply the diffusion equation to Fig. 1. (a) Draw the shape for C(z) vs I just after partition removal (i.e. the gas totally in the left com- partment), and indicate flux J, at the three 2-positions indicated. Please do the following: Make your axes like that shown in Fig. 3. The vertical blue dashed lines are the three : locations you should indicate flux Jz. Indicate J, with an arrow with the proper direction and magnitude. C(x) 0 Figure 3: Example of axes you should use for Problem 5 (b) Draw the shape for C() vs I when the new equilibrium state has been achieved; then, in the same way you did for part (a), indicate the flux J, at 2-positions indicated by the vertical (dashed) lines. VS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


