Question: Now, we are going to use our spectroscopy knowledge for the most common real-life case; identifying an element! Let us imagine that you are performing
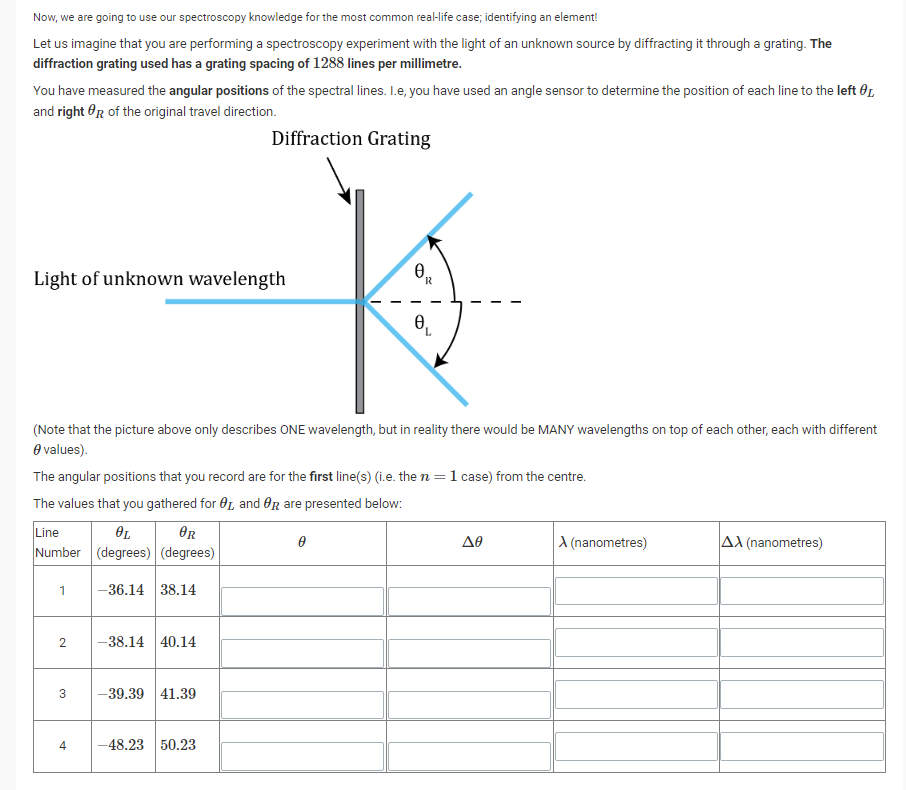
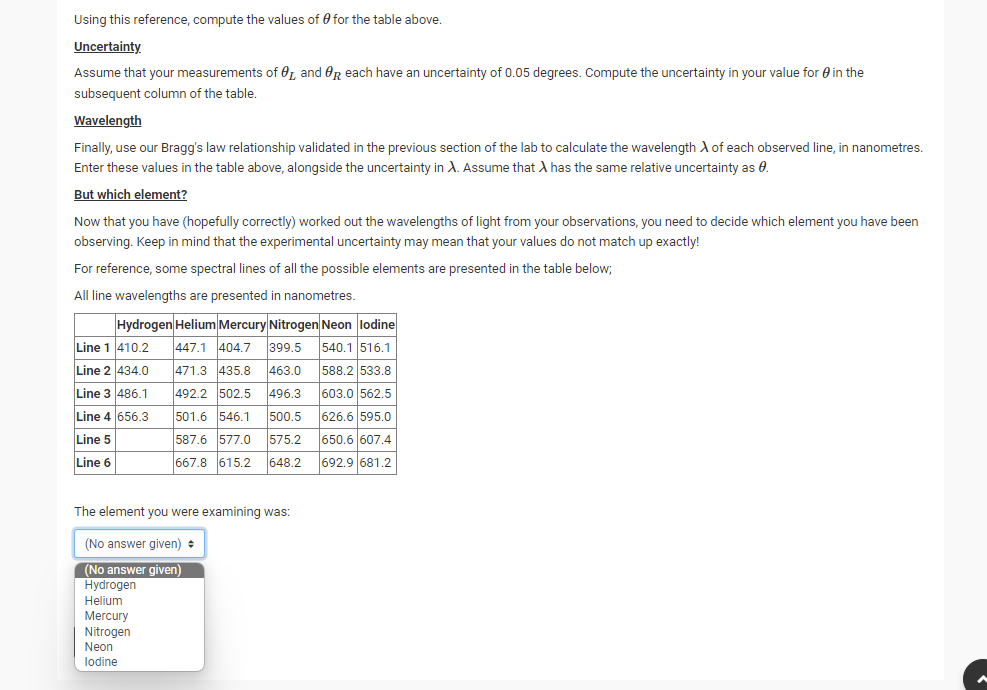
Now, we are going to use our spectroscopy knowledge for the most common real-life case; identifying an element! Let us imagine that you are performing a spectroscopy experiment with the light of an unknown source by diffracting it through a grating. The diffraction grating used has a grating spacing of 1288 lines per millimetre. You have measured the angular positions of the spectral lines. I.e, you have used an angle sensor to determine the position of each line to the left Of and right OR of the original travel direction. Diffraction Grating Light of unknown wavelength (Note that the picture above only describes ONE wavelength, but in reality there would be MANY wavelengths on top of each other, each with different 9 values). The angular positions that you record are for the first line(s) (i.e. the n = 1 case) from the centre. The values that you gathered for Or, and Op are presented below: Line A (nanometres) AX (nanometres) Number (degrees) | (degrees 36.14 38.14 2 -38.14 40.14 3 -39.39 41.39 4 48.23 50.23Using this reference, compute the values of Efor the table above. Uncertainty Assume that your measurements of 91, and HR each have an uncertainty of 0.05 degrees. Compute the uncertainty in your value for 6' in the subsequent column of the table. Wavelength Finally, use our Bragg's lav.r relationship validated in the previous section ofthe lab to calculate the wavelength A. of each observed line, in nanometres. Enter these values in the table above, alongside the uncertainty in 1. Assume that A has the same relative uncertainty as I9. But which element? Now that you have (hopefully correctly} worked out the wavelengths of light from your observations, you need to decide which element you have been observing. Keep in mind that the experimental uncertainty may mean that your values do not match up exactly! For reference, some spectral lines of all the possible elements are presented in the table below, All line wavelengths are presented in nanometres. Hydrogen l-leliun Mermry Nitmgen Neon Iodine Line 1 410.2 4411 4-04.? 399.5 540.1 516.1 Line 2 434.0 4?1.3 435.8 463.0 583.2 533.8 Line3 436.1 492.2 502.5 496.3 603.0 562.5 Lineb 6613 615.2 643.2 692.9 681.2 The element you were examining was: [No answer given} : {No answer given}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


