Question: number 16 please How much time (in s) is needed for NOCl originally at a concentration of 0.0136M to decay to 0.0039M ? Answer: Question

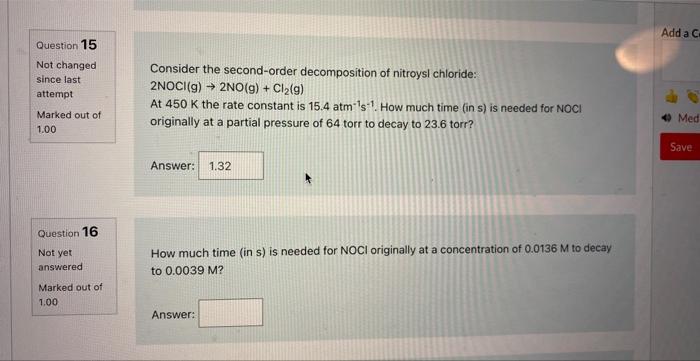
How much time (in s) is needed for NOCl originally at a concentration of 0.0136M to decay to 0.0039M ? Answer: Question 15 Not changed Consider the second-order decomposition of nitroysl chloride: since last attempt 2NOCl(g)2NO(g)+Cl2(g) At 450K the rate constant is 15.4atm1s1. How much time (in s) is needed for NOCl originally at a partial pressure of 64 torr to decay to 23.6 torr? Answer: How much time (in s) is needed for NOCl originally at a concentration of 0.0136M to decay to 0.0039M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


