Question: 3. Assume that the density of positive charge in any Thomson atom is the same as for the hydrogen atom. Find the radius R

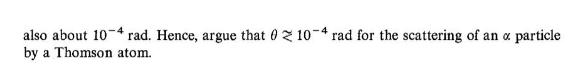
3. Assume that the density of positive charge in any Thomson atom is the same as for the hydrogen atom. Find the radius R of a Thomson atom of atomic number Z in terms of the radius RH of the hydrogen atom. 4. (a) An a particle of initial velocity v collides with a free electron at rest. Show that, as- suming the mass of the a particle to be about 7400 electronic masses, the maximum de- flection of the a particle is about 10 rad. (b) Show that the maximum deflection of an a particle that interacts with the positive charge of a Thomson atom of radius 1.0 is also about 10-4 rad. Hence, argue that 0 z 10-4 rad for the scattering of an a particle by a Thomson atom.
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Solution for Question 3 Given Density of positive charge in any Thomson atom density in hydrogen ato... View full answer

Get step-by-step solutions from verified subject matter experts


