Question: number 3, part 2 & 3. The gas sample has now returned to its original state of 1.00atm,20.0C and 1.00L. What will the pressure become
number 3, part 2 & 3.
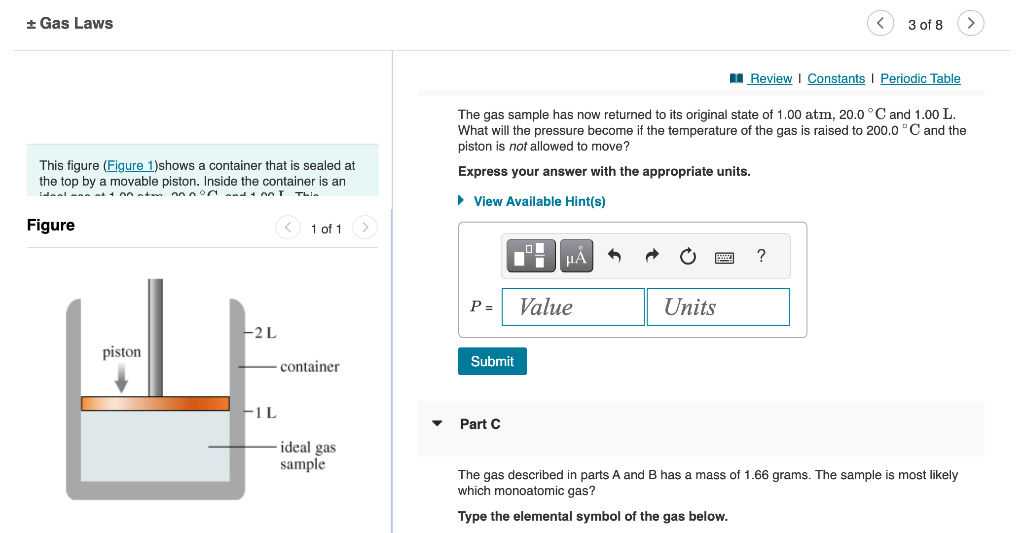
The gas sample has now returned to its original state of 1.00atm,20.0C and 1.00L. What will the pressure become if the temperature of the gas is raised to 200.0C and the piston is not allowed to move? This figure Ishows a container that is sealed at the top by a movable piston. Inside the container is an Express your answer with the appropriate units. Figure 1 of 1 Part C The gas described in parts A and B has a mass of 1.66 grams. The sample is most likely which monoatomic gas? Type the elemental symbol of the gas below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


