Question: o) Codeine (C18H21NO3) is a weak organic base. A5.0103M solution of codeine has a pH of 9.95. C18H21NO3(aq)+H2O(l)C18H21NO3H+(aq)+OH(aq) i) Calculate the equilibrium concentration of C18H21NO3H+(aq).
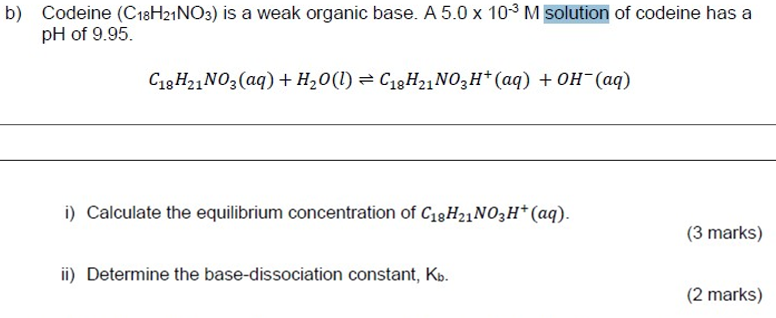
o) Codeine (C18H21NO3) is a weak organic base. A5.0103M solution of codeine has a pH of 9.95. C18H21NO3(aq)+H2O(l)C18H21NO3H+(aq)+OH(aq) i) Calculate the equilibrium concentration of C18H21NO3H+(aq). (3 marks) ii) Determine the base-dissociation constant, Kb. (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


