Question: Objective: This activity has the purpose of helping students explain what a buffer solution is, prepare a buffer solution and interpret a titration curve. Student
Objective:
This activity has the purpose of helping students explain what a buffer solution is, prepare a buffer solution and interpret a titration curve.
Student Instructions:
Solve the following problems on paper.
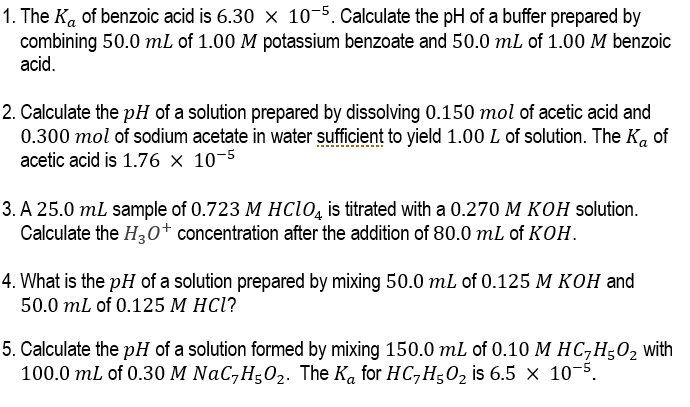
1. The Ka of benzoic acid is 6.30105. Calculate the pH of a buffer prepared by combining 50.0mL of 1.00M potassium benzoate and 50.0mL of 1.00M benzoic acid. 2. Calculate the pH of a solution prepared by dissolving 0.150mol of acetic acid and 0.300mol of sodium acetate in water sufficient to yield 1.00L of solution. The Ka of acetic acid is 1.76105 3. A 25.0mL sample of 0.723MHClO4 is titrated with a 0.270MKOH solution. Calculate the H3O+concentration after the addition of 80.0mL of KOH. 4. What is the pH of a solution prepared by mixing 50.0mL of 0.125MKOH and 50.0mL of 0.125MHCl ? 5. Calculate the pH of a solution formed by mixing 150.0mL of 0.10MHC7H5O2 with 100.0 mL of 0.30MNaC7H5O2. The Ka for HC7H5O2 is 6.5105
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


