Question: observation sheet extra observations : volume for run 1 will be 9.86ml please convert to L when doing work question- this graph below is for
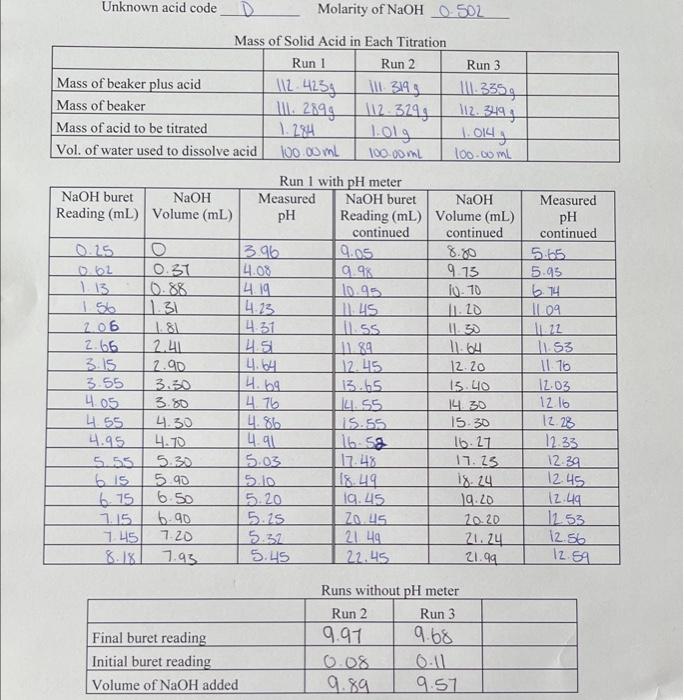
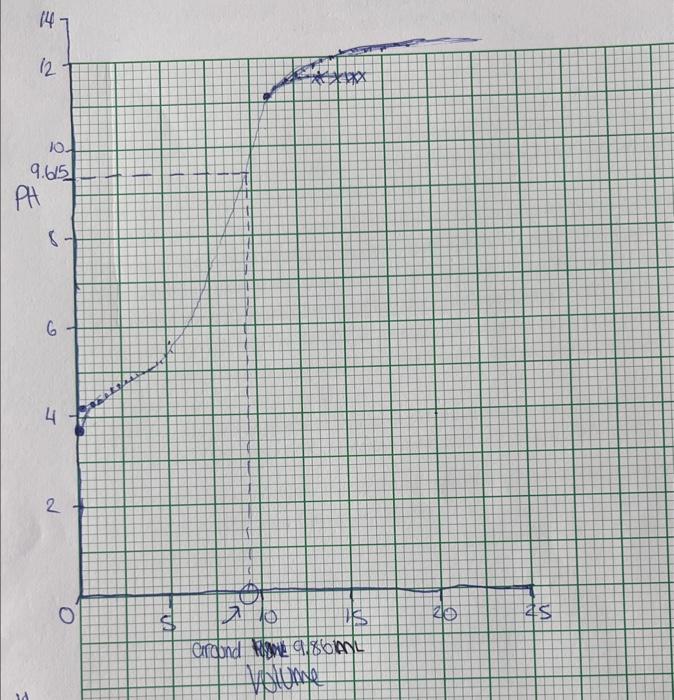

Mace of Solid A oid in Fanh Titmation D Dun 1 wish ot watao 2. For each titration, calculate the moles of base needed to neutralize the acid sample. Give your calculation for Run 1 here: ( 0.5 mark) Calculation for Run 1: Run number moles of NaOH needed C=vn0.502M=0.00980Lmol Mal NaOH=0.00495 (a) Enter your unknown acid code D_. For each run, calculate the molar mass of your unknown acid. Show your calculation for Run 1 here: ( 0.5 mark) Calculation for Run 1: Run number value of molar mass (g/mol) (b) Based on your OWN results, give the value of the molar mass that you want to be marked : (3 marks) Mace of Solid A oid in Fanh Titmation D Dun 1 wish ot watao 2. For each titration, calculate the moles of base needed to neutralize the acid sample. Give your calculation for Run 1 here: ( 0.5 mark) Calculation for Run 1: Run number moles of NaOH needed C=vn0.502M=0.00980Lmol Mal NaOH=0.00495 (a) Enter your unknown acid code D_. For each run, calculate the molar mass of your unknown acid. Show your calculation for Run 1 here: ( 0.5 mark) Calculation for Run 1: Run number value of molar mass (g/mol) (b) Based on your OWN results, give the value of the molar mass that you want to be marked
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


