Question: OH group Pentanol molecule Hexane molecule How do you think the OH on the end of the pentanol molecule will affect the amount of surface
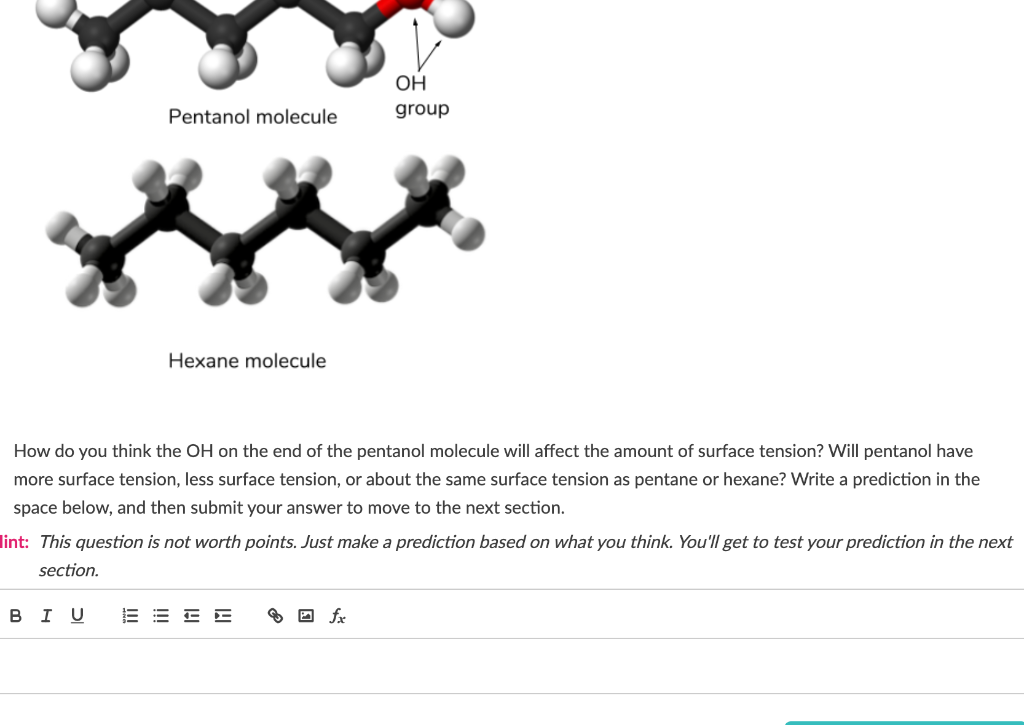
OH group Pentanol molecule Hexane molecule How do you think the OH on the end of the pentanol molecule will affect the amount of surface tension? Will pentanol have more surface tension, less surface tension, or about the same surface tension as pentane or hexane? Write a prediction in the space below, and then submit your answer to move to the next section. lint: This question is not worth points. Just make a prediction based on what you think. You'll get to test your prediction in the next
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


