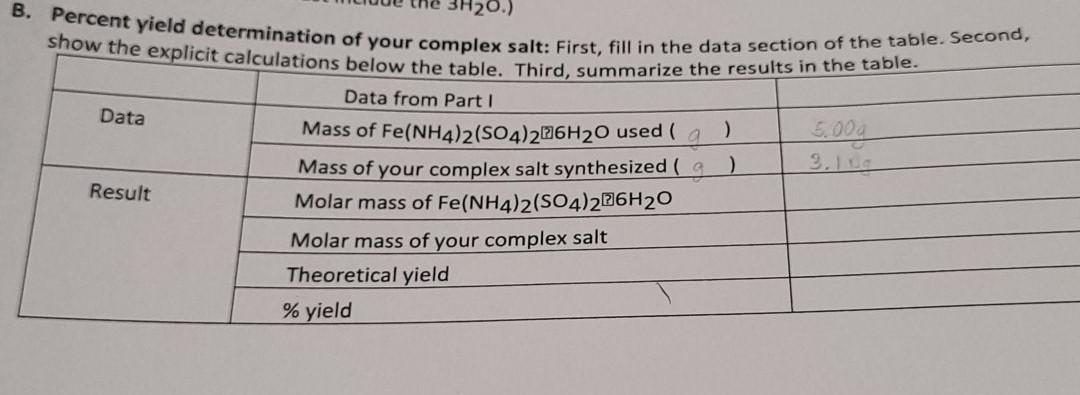
Question: Old MathJax webview Formula:K11Fe(C2O4)2 3H2O Data B. Percent yield determination of your complex salt: First, fill in the data section of the table. Second, show
Old MathJax webview

Formula:K11Fe(C2O4)23H2O
Data B. Percent yield determination of your complex salt: First, fill in the data section of the table. Second, show the explicit calculations below the table. Third, summarize the results in the table. Data from Part I Mass of Fe(NH4)2(SO4)226H20 used ) 5,000 Mass of your complex salt synthesized ( ) Result Molar mass of Fe(NH4)2(SO4)206H20 Molar mass of your complex salt Theoretical yield % yield EXPERIMENT 5 1. Show the molar mass calculation for Fe(NH4)2(SO4)2.6H20. (Remember to include the mass of the 6H2O molecules.) 2. Show the molar mass calculation of your complex salt (Remember to include the mass of the 3H20 molecules.) 3. Show the calculation of the theoretical yield of the complex salt, using the measured mass of the iron salt you started with in the synthesis, Fe(NH4)2(SO4)2.6H20, and the fact that all of the iron(11) ions in the reactant theoretically end up in the final product. 4. Show calculation of the percent yield of complex salt in your synthesis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


