Question: Old MathJax webview Old MathJax webview d. Calculate the total mmole HCl in the acid trap e. Calculate the amount (mmoles) of excess HCl that
Old MathJax webview
d. Calculate the total mmole HCl in the acid trap e. Calculate the amount (mmoles) of excess HCl that was back titrated with NaOH
Please disregard the first picture and the 2 questions below it
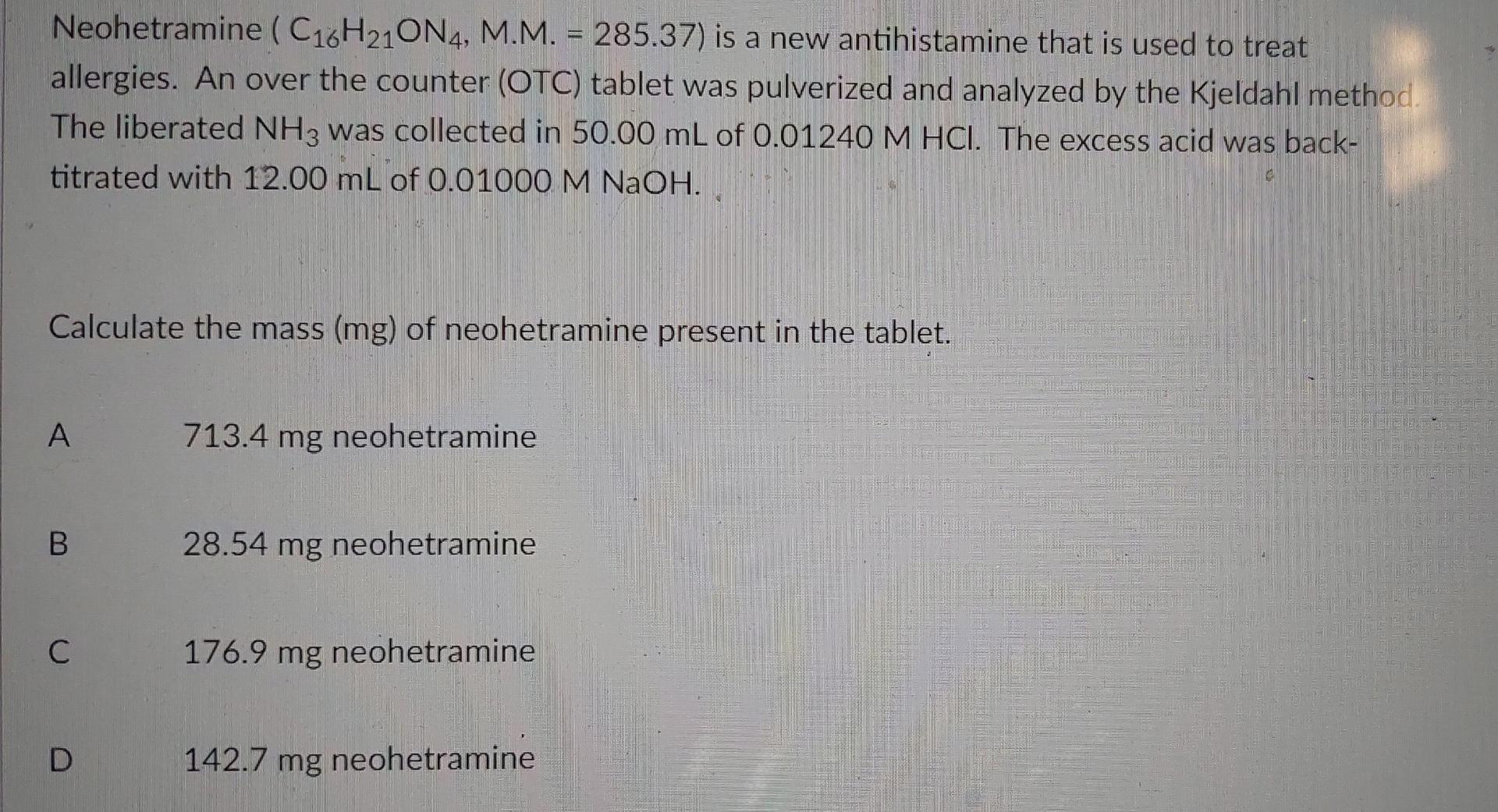
Neohetramine (C16H210N4, M.M. = 285.37) is a new antihistamine that is used to treat allergies. An over the counter (OTC) tablet was pulverized and analyzed by the Kjeldahl method The liberated NH3 was collected in 50.00 mL of 0.01240 M HCl. The excess acid was back- titrated with 12.00 mL of 0.01000 M NaOH. Calculate the mass (mg) of neohetramine present in the tablet. A . 713.4 mg neohetramine B 28.54 mg neohetramine C 176.9 mg neohetramine 142.7 mg neohetramine
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


