Question: Old MathJax webview you can skip it plz dont copy paste otherwise i report sure upvote mouills are given in Table 2.1 below: Table 2.1.
Old MathJax webview
you can skip it plz dont copy paste otherwise i report
sure upvote
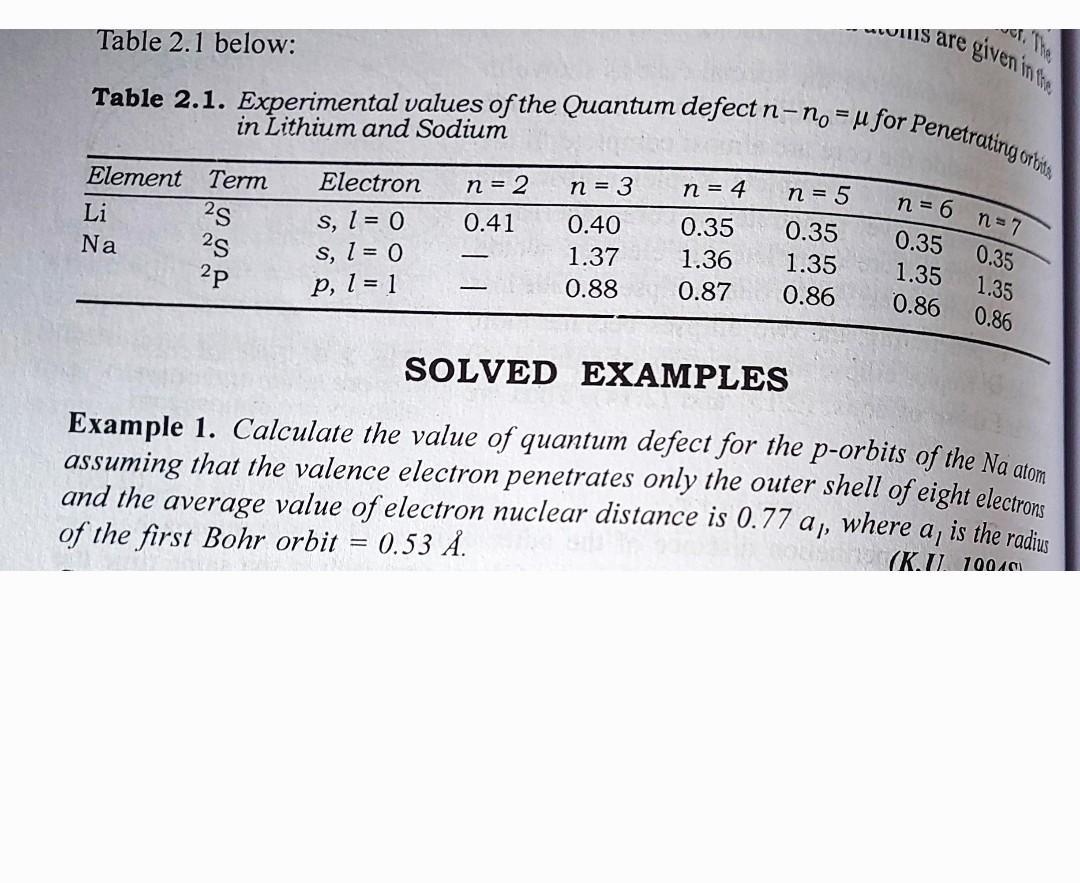
mouills are given in Table 2.1 below: Table 2.1. Experimental values of the Quantum defect n-no = u for Penetrating orbits in Lithium and Sodium Electron n = 2 n = 3 n = 4 n = 5 Element Term Li 2s Na 2s 2P 0.41 S, I = 0 S, I = 0 P, 1 = 1 0.40 1.37 0.88 0.35 1.36 0.87 0.35 1.35 0.86 0.35 1.35 0.86 n= 6 n=7 0.35 1.35 0.86 SOLVED EXAMPLES Example 1. Calculate the value of quantum defect for the p-orbits of the Na atom assuming that the valence electron penetrates only the outer shell of eight electrons and the average value of electron nuclear distance is 0.77 an, where of the first Bohr orbit 0.53 . is the radius al (K.U. 100 101
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


