On another planet, the isotopes of titanium have the given natural abundances. Abundance Mass (u) 72.400%...
Fantastic news! We've Found the answer you've been seeking!
Question:
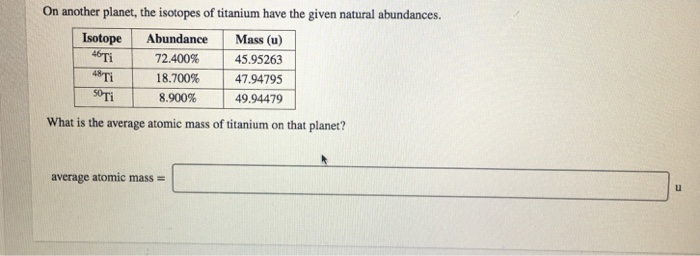
Transcribed Image Text:
On another planet, the isotopes of titanium have the given natural abundances. Abundance Mass (u) 72.400% 45.95263 18.700% 47.94795 8.900% 49.94479 Isotope 46 Ti 48 Ti 50 Ti What is the average atomic mass of titanium on that planet? average atomic mass= u On another planet, the isotopes of titanium have the given natural abundances. Abundance Mass (u) 72.400% 45.95263 18.700% 47.94795 8.900% 49.94479 Isotope 46 Ti 48 Ti 50 Ti What is the average atomic mass of titanium on that planet? average atomic mass= u
Expert Answer:
Answer rating: 100% (QA)
Average atomic mass of the Titanium is 4668104 Solution The average atomic ... View the full answer

Related Book For 

Posted Date:
Students also viewed these chemistry questions
-
The natural abundances of the two stable isotopes of hydrogen (hydrogen and deuterium) are 11H: 99.985 percent and 21H: 0.015 percent. Assume that water exists as either H2O or D2O. Calculate the...
-
Natural carbon, which has an atomic mass of 12.011 amu, consists of carbon-12 and carbon 13 isotopes. Given that the mass of carbon-13 is 13.00335 amu, what would be the average atomic mass (in amu)...
-
Natural chlorine, which has an atomic mass of 35.4527 amu, consists of chlorine-35 and chlorine-37 isotopes. Given that the mass of chlorine-35 is 34.96885 amu, what is the average atomic mass (in...
-
In a recent survey, 80% of the community favored building a police substation in their neighborhood. If 20 citizens are chosen, what is the mean and standard deviation for the number favoring the...
-
List some common examples of principal and income items under the Uniform Act.
-
Define the terms Idealism. Use the 4-step methodology of understanding, analysis, evaluation and application to develop your thesis. Provide arguments both in support of and in opposition to your...
-
Suppose \(\mathbf{x} \sim M N(n, \boldsymbol{\pi})\) follows a multinomial distribution of size \(n\) and probability \(\pi\). Derive the variance matrix of \(\mathbf{x}\).
-
Large Lots is planning a seven-day promotion on a discontinued model of 31" color television sets. At a price of $575 per set, the daily demand for this type of TV has been estimated as follows:...
-
Solving via linear equation No unread replies.No replies. A small country exports soybeans and flowers. Soybeans require 8 workers per acre, flowers require 12 workers per acre, and 100,000 workers...
-
Jiminys Cricket Farm issued a 30-year, 4.5 percent semiannual bond three years ago. The bond currently sells for 104 percent of its face value. The companys tax rate is 22 percent. a. What is the...
-
Suppose we are testing a null hypothesis, and the p-value associated with the observed test statistic is p=0.047. Would you reject the null hypothesis under a 1% significance level? Under a 5%...
-
Give three reasons why an organization may support projects that do not have high-profit margins, also explain. Discuss how project selection might be different in an agile versus a plan-driven or...
-
Q. What is VPN Tunneling? Explain it's Types.
-
Review the Case of Maria . come up with at least 2 SMART goals for Maria's treatment plan. Use Ch. 8 of the text to create goals that are specific, measurable, achievable, realistic, and time-phased....
-
An object with a mass of 500 kg starts to move from rest and travels 8 m upwards on an inclined plane in 15 s. Since the kinetic friction coefficient between the crate and the inclined plane is =...
-
By now you have chosen your topic for your business presentation and given your elevator pitch. Please share your thoughts on the following questions. Is it more challenging to give a presentation...
-
A teacher gives her class a particular examination on mathematics at the end of each year. The results are normally distributed with a mean test score of 63 and a standard deviation of 8. The teacher...
-
Juanita owns a home in Richardson, TX. She purchases a Homeowners Policy (HO-3) from Farm State Ins. Co. The policy provides $100,000 in liability coverage (coverage E) and $5,000 in Med Pay coverage...
-
A solution containing 0.8330 g of a polymer of unknown structure in 170.0 mL of an organic solvent was found to have an osmotic pressure of 5.20 mmHg at 25C. Determine the molar mass of the polymer.
-
Beginning with 3-methyl-1-butyne, show how you would prepare the following compounds: (a) (b) (c) Br CH3 CH2=C-CH-CH CH3 CH2Br--CBr2-CH-CH3 Br CH3 CH3-CH-CH CH
-
The radioactive isotope 238Pu, used in pacemakers, decays by emitting an alpha particle with a half-life of 86 yr. (a) Write an equation for the decay process. (b) The energy of the emitted alpha...
-
Find the state vector via the formal-solution approach. \(\dot{\mathbf{x}}=\left[\begin{array}{cc}5 & 1 \\ -4 & 1\end{array} ight] \mathbf{x}+\left[\begin{array}{c}1 \\ -1\end{array} ight] u, \quad...
-
A nonlinear dynamic system is described by a. Use the Simulink model to plot x 2 ( t ) x 2 ( t ) . b. Derive the linearized model analytically, build its Simulink model where the linear model is...
-
Consider the mechanical system in Figure 8.28. Assuming \(m=12 \mathrm{~kg}\), \(b=20 \mathrm{~N}\)-sec \(/ \mathrm{m}, k=200 \mathrm{~N} / \mathrm{m}, F_{0}=47 \mathrm{~N}\), and \(\omega=5...

Study smarter with the SolutionInn App


