Question: The student performs a second titration using the 0.10M NaOH(aq) solution again as the titrant, but this time with a 20.mL sample of 0.20MHCl(aq) instead
 The student performs a second titration using the 0.10M NaOH(aq) solution again as the titrant, but this time with a 20.mL sample of 0.20MHCl(aq) instead of 0.10MHCl(aq).
The student performs a second titration using the 0.10M NaOH(aq) solution again as the titrant, but this time with a 20.mL sample of 0.20MHCl(aq) instead of 0.10MHCl(aq).
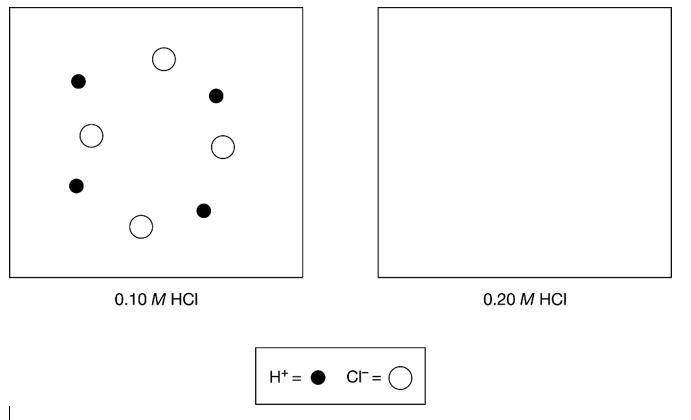
1. The box below to the left represents ions in a certain volume of 0.10M HCl(aq). In the box below to the right, draw a representation of ions in the same volume of 0.20MHCl(aq). (Do not include any water molecules in your drawing.).
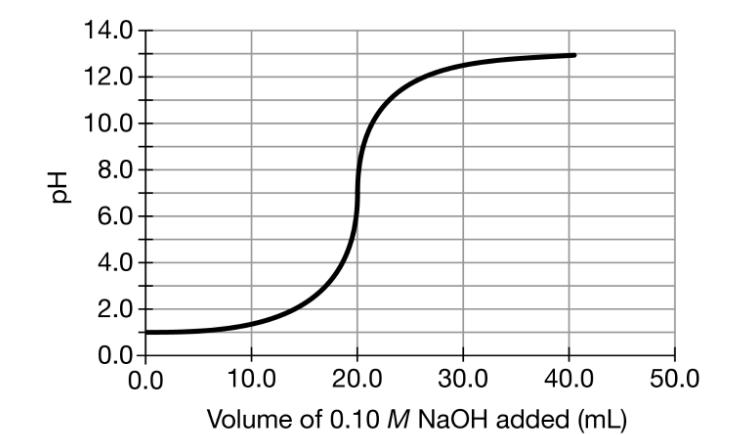
2. graph in part (d), carefully draw a curve that shows the results of the second titration, in which the student titrates a 20.mL sample of 0.20MHCl(aq) with 0.10MNaOH(aq).
14.0 12.0 10.0 8.0 6.0 4.0 2.0 0.0+ 0.0 10.0 20.0 30.0 40.0 50.0 Volume of 0.10 M NaOH added (mL) Hd
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Answer Heres what I get Explanation g Titration curves I ... View full answer

Get step-by-step solutions from verified subject matter experts


