Question: One method to obtain pure Silicon is through a process where the following reactions: SiO2 + 2 C ------ Si +2 CO Si+ 2 Cl2
One method to obtain pure Silicon is through a process where the following reactions:
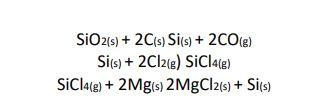
SiO2 + 2 C ------ Si +2 CO
Si+ 2 Cl2 ----- SiCl4
SiCl4+ 2 Mg ---- 2 MgCl2 + Si
In one particular case, 100 pounds of a solid mixture containing 51.94% SiO2, 15.58% C and 32.48% Mg together with 0.13 mol-lb of Chlorine gas (Cl2), obtaining as a product 76.05 pounds of solid product and a gaseous mixture whose molar composition is 2.28% Cl2, 1.21% SiCl4, and 96.51% CO. Find: i) The percentage excess of the reaction. ii) The conversion of the three reactions. iii) The weight composition of the final solid product. iv) The pounds of silicon produced.
Hello, the question is complete, they ask me to work with this data and information
I enclose as you see in the work guide, I'm sorry but it is in Spanish

SiO2 (s) + 2C(s) Si(s) + 2CO(g) Si(s) + 2Cl2(g) SiCl4(g) SiCl4(g) + 2Mg(s) 2MgCl2(s) + Si(s) 4. Un mtodo para obtener Silicio puro es a travs de un proceso en donde se producen las siguientes reacciones: SiO2(s) + 2C(s) S(s) + 2CO(g) Si(s) + 2Cl2(g) SiCl4(g) SiCl4(g) + 2Mg(s) 2MgCl2(s) + Si(s) En un caso determinado, al proceso se alimentan 100 libras de una mezcla slida que contiene 51.94% de SiO2, 15.58 % de C y 32.48% de Mg junto con 0.13 moles-lb de gas Cloro (Cl2), obtenindose como producto 76.05 libras de producto slido y una mezcla gaseosa cuya composicin molar es 2.28% Cl2, 1.21% SiCl4 y 96.51% de CO. Encuentre: i) El porcentaje de exceso de la reaccin. ii) La conversin de las tres reacciones. iii) La composicin en peso del producto slido final. iv) Las libras de silicio producidas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


