Question: one question Q5. Data were collected to determine the rate law for the reaction of potassium iodide with hydrogen peroxide. The following data were collected
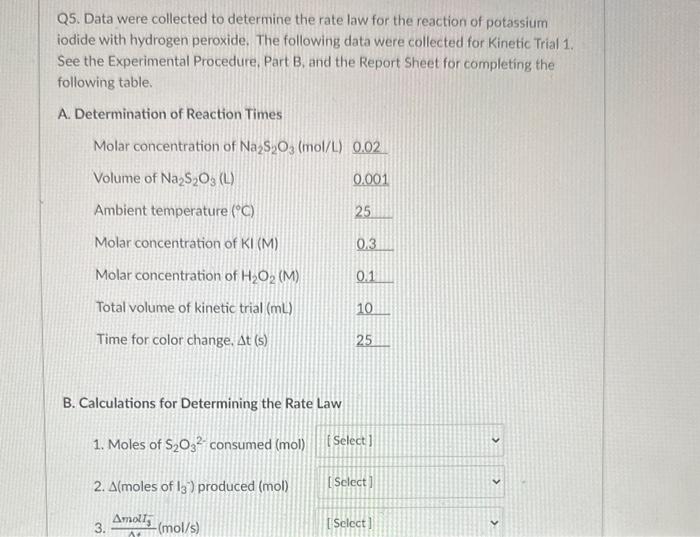
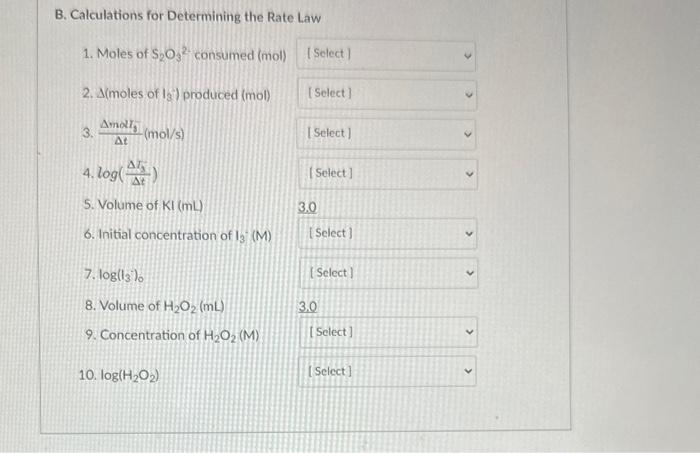
Q5. Data were collected to determine the rate law for the reaction of potassium iodide with hydrogen peroxide. The following data were collected for Kinetic Trial 1. See the Experimental Procedure, Part B, and the Report Sheet for completing the following table. B. Calculations for Determining the Rate Law 1. Moles of S2O32 consumed (mol) 2. ( moles of I3) produced (mol) 3. tmol5(mol/s) B. Calculations for Determining the Rate Law 1. Moles of S2O32 consumed (mol) 2. A (moles of I3) produced (mol) 3. tmat3(mol/s) 4. log(t3) 5. Volume of KI(mL) 3.0. 6. Initial concentration of I3(M) 7. log(13)0 8. Volume of H2O2(mL) 3.0 9. Concentration of H2O2 (M) 10. log(H2O2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


